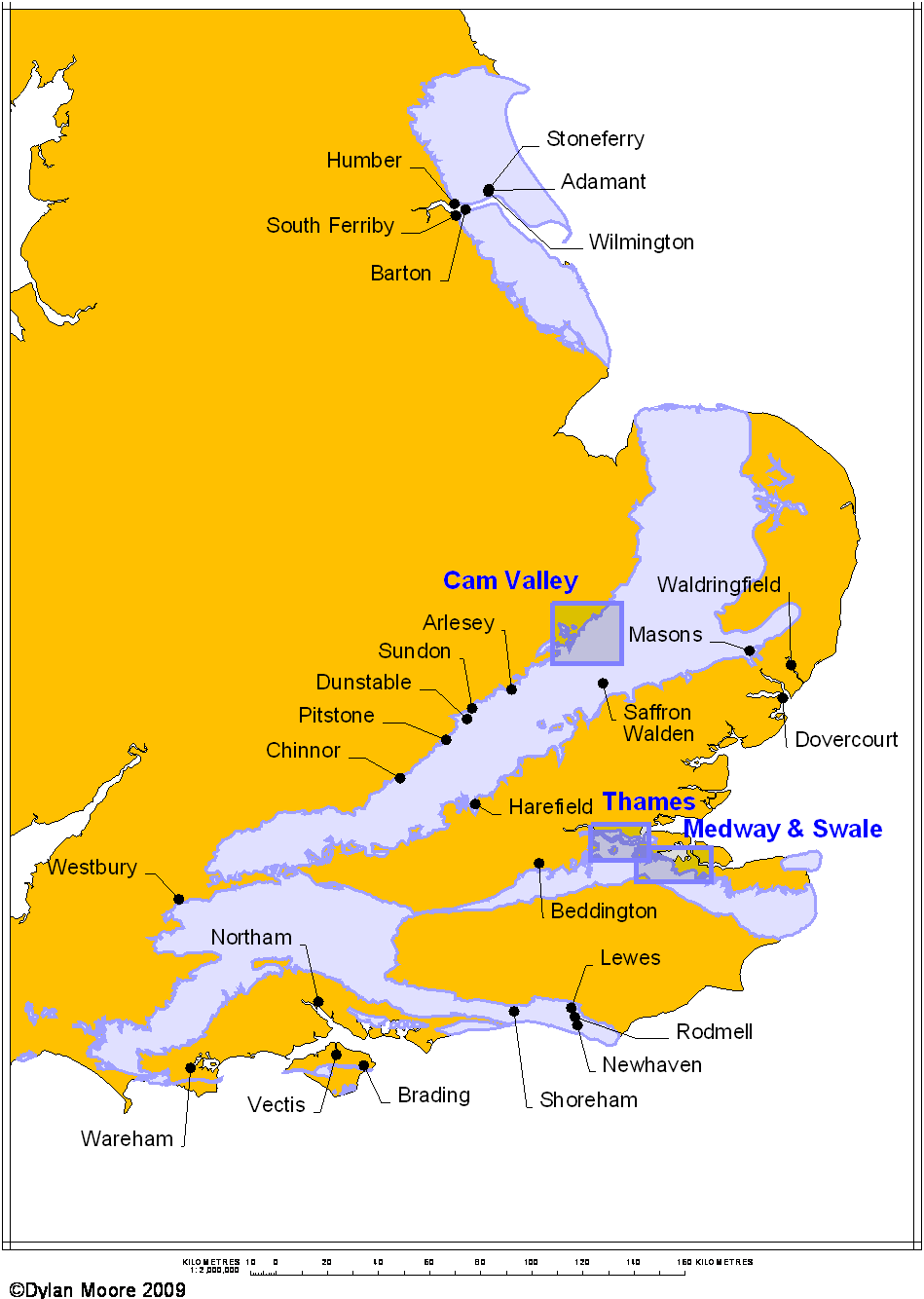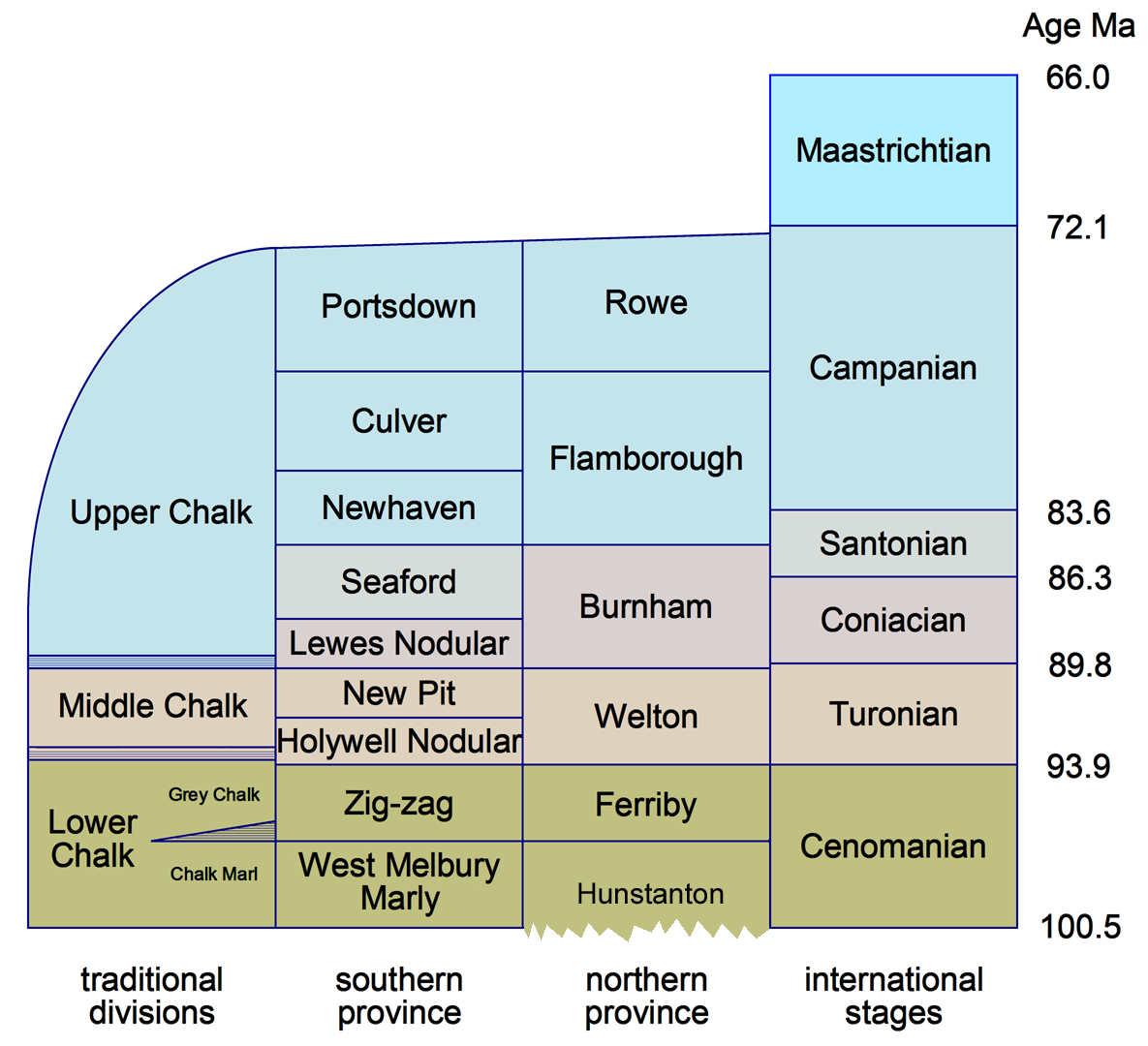
The modern designations of Chalk Formations are divided into two provinces, the northern beginning in Norfolk, somewhat south of the Wash. In the northern province, the Hunstanton Formation represents a condensed part of the Lower Cenomanian and the Albian and Aptian (part of the Lower Cretaceous), and typically takes the form of the "Red Chalk".
The British Chalk Formation occupies all six internationally-defined stages of the Upper Cretaceous. Traditionally, and still to some extent on BGS mapping, the chalk is divided into Lower, Middle and Upper divisions, divided at the hard bands that give rise to the chalk escarpment features of south-east England. The Lower Chalk consists of most of the Cenomanian. The Middle Chalk has as its base a hard band most often known as the Melbourne Rock, includes the whole of the Turonian. The Upper Chalk has as its base a hard band most commonly called the Chalk Rock, which is the base member of the Coniacian and forms the lip of all the main chalk escarpments in southern England. The rest of the Upper Chalk occupies the dip slope of the escarpments. The three highest Cretaceous stages have mostly been eroded away, and the youngest British chalk, found only in a small area on the Norfolk coast, is Lower Maastrichtian.
Of the three divisions, the Lower Chalk is most variable, and is conventionally divided into four lithological subdivisions, as commonly found in the Chilterns: at the base the Glauconitic Marl, which is a thin green transitional bed, often sandy and/or phosphatic. Above this is the Chalk Marl, which is a bluish argillaceous limestone containing 50-80% calcium carbonate, and next the Grey Chalk, which is also argillaceous, containing 80-95% calcium carbonate. At the top are the very persistent Plenus Marls, typically a thin succession of yellow and dark grey marls. In a region between Buckinghamshire and Norfolk, the base of the Grey Chalk consists of a prominent hard band most commonly called the Totternhoe Stone, which sometimes produces a third, distinct chalk escarpment.
The Lower Chalk (which, minus the Plenus Marls, corresponds to the Cenomanian stage of the Cretaceous) is quite distinct from the later stages in its geological history. It was laid down in generally shallower sea conditions, with cyclic variations in depth. A lowering of sea level brought argillaceous sediments further out, and encouraged their production by rejuvenation. Many rhythms can be identified as Milankovich cycles. Occasionally sea levels fell low enough for deposition to cease and erosion set in. These events encouraged the formation of nodular concretions of various minerals such as pyrite and apatite. The overall effect is that the carbonate content is variable, with bands of impure marl interspersed with relatively pure chalk.
Almost everywhere, the lower layers of the Lower Chalk have successively lower calcium carbonate contents, often dropping to as low as 50% at the base. These have been used for centuries to make hydraulic limes, and many attempts were made to make them into “Natural Cements”. With addition of some higher-grade chalk, they have been used to make cements using only chalk as raw material, allowing a greatly-simplified process.
The Cenomanian chalk of the Chiltern Hills links the northern and southern chalk provinces and its chemistry is treated in more depth in an article on the Chilterns.
The Middle and Upper Chalks are both relatively pure calcium carbonate, apart from the occurrence of flint nodules. In the south, flint is found only in the Upper Chalk, where it may comprise up to 10% of the rock. The flint nodules, being extremely hard, can readily be removed undamaged from the chalk matrix by gentle wet grinding. Many of the larger cement plants developed flint sales as a sideline - the larger (and therefore more pure) flints were sold to the pottery industry for use, after calcination, as a tempering agent in ceramics. North of the Wash, the situation is reversed, with Upper Chalk containing relatively little flint, while flint is fairly abundant in the Middle Chalk.
Aside from a few percent of non-carbonate materials, the chalk is almost entirely calcite, which is almost pure calcium carbonate, with small amounts (usually less than 0.1% each) of foreign ions replacing calcium: mainly Mg, Mn(II), Fe(II) and Sr.
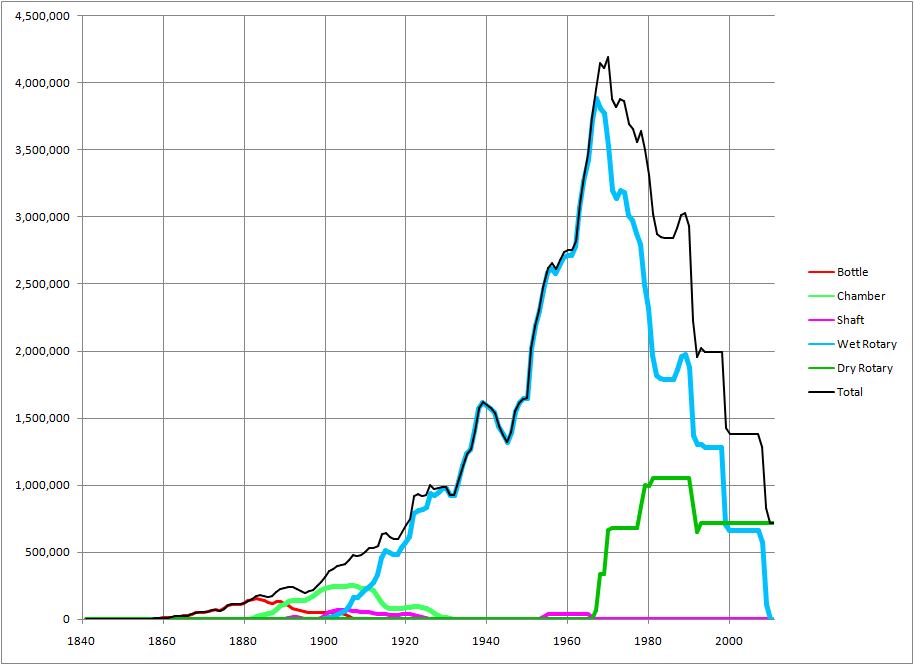
The softness of chalk made it a natural choice of raw material for the early cement industry, when cement manufacturing technology was primarily limited by lack of effective grinding techniques. The fine grinding of hard materials was initially at best expensive, and at worst impossible. A second, more subtle reason for the early preference for chalk was its chemical dependability, amenable to the use of simple non-technical rules-of-thumb. If it’s white, it’s pure calcium carbonate. If it’s grey, it’s argillaceous, but still low in MgO and alkalis. All the other limestones are mineralogically variable, prone to be dolomitic, argillaceous or siliceous, and colour is no sure guide to these variations. Early attempts to use such limestones for cement were no doubt ended by nasty and unaccountable surprises. Their effective use required the services of a professional chemist, and 24-hour chemical monitoring. Before about 1890, this was an almost insuperable obstacle. The high moisture content of chalk has militated against its use in modern energy-efficient dry process plants, so the industry which initially used chalk almost exclusively because of its softness, has now disappeared in southeast England, because of its intrinsic energy cost.
Although some plants using Chalk Marl needed no other secondary raw material, in general some sort of clay needed to be used in conjunction with the high purity chalks. Initially, the silty alluvial clays of the Thames and Medway estuaries were favoured, for a number of reasons: they could be obtained from the foreshore, avoiding mineral royalty costs; by their nature, they were amenable to water transport; and they contained plenty of fine-grained silica allowing easy burning. However, they were also necessarily high in salt content, which is unfavourable for use in rotary kilns, and so alternative clays were sought. In the London Basin, Eocene London Clay usually outcrops fairly close to the chalk quarries, while plants on the scarp slopes of the chalk tended to use the underlying Cretaceous Gault Clay or clays of the Upper Jurassic such as the Kimmeridge Clay.