Portland cement rawmix usually contains between 76 and 79% of calcium carbonate. When the main raw material is a limestone containing more than this amount of calcium carbonate, the composition must be adjusted by addition of a low-carbonate component, contributing SiO2 (silica), Al2O3 (alumina) and Fe2O3 (iron oxide) to the mixture. The aluminosilicate-bearing minerals used are referred to as Argillaceous (clay-like) Components.
In some instances, cement manufacturers have had access to "argillaceous limestones" that have carbonate contents close to the target level - the most commonly used in Britain being the Chalk Marl and the Blue Lias. In these cases, rawmix control consisted of blending higher-carbonate and lower-carbonate layers to obtain the exact target chemistry. However, in general, nature is not that kind. The main raw material may be somewhat too low in carbonate, in which case a high-carbonate "sweetener" must be obtained to correct the mix. But in the majority of cases, the main limestone raw material is relatively pure, and must be blended with a clay or shale that is low in calcium carbonate. The nature of this "secondary material" chosen changed with the historical evolution of the industry.
The clays and shales used are discussed here in increasing order of geological age.
Alluvium
Alluvium is mud carried by and deposited by rivers, typically during the last few thousand years. Alluvial clay was the first to be used in Portland cement in the Thames valley (and although the source of clay used by Joseph Aspdin in Yorkshire is unknown, it was most probably also alluvium). Early cement manufacturers ascribed almost magical qualities to the alluvium of the Medway estuary, and even distant plants (e.g. Shoreham) used it, obtaining supplies by coastal barge. The key quality of the clay, not identified until much later, was the content of fine silica, in addition to clay minerals, and the existence of highly-prized areas of the estuary in which large particles were absent due to the slow river currents and tidal flows transporting the sediment.
The popularity of the Medway alluvium resulted in a large specialised industry supplying clay to cement plants, by a method not generally employed elsewhere: clay was hand-dug from the foreshore of the very wide and meandering estuary, and conveyed by barges that arrived at high tide, beached as the tide fell, were filled at low tide, and floated off as the tide rose again. This industry considerably changed the estuary, removing most of the salt-marsh that had previously delineated the river's course. The product of this extraction method was naturally very wet (>40% as dug), and had a high content of salt, organics (up to 5%) and pyrite. Salinity in most of the worked area of the estuary was around 2.7-2.9%, so a clay with 45% as-dug water content (i.e. 81.8% dry basis) contained around 2.3% sodium chloride. Neither the moisture nor the salt were a problem for the early industry, preparing high moisture content (>60%) slurries, removing most of the water (plus dissolved salt) by decantation, and burning in a static kiln that evaporated all salts.
Later manufacturers, looking for materials closer to hand, soon found that many other alluvial clays were suitable, beginning with the clays of the Thames estuary. In many locations there were salt marshes yielding good clay, and furthermore, these were above water level at all but the highest spring tides, and so could be quarried by conventional means. The largest such deposits were at Cliffe, which for a while supplied many of the Thames plants. Clay was dug from the flooded workings by means of a floating dredger equipped with an on-board washmill that slurried the clay as it was dug, and pumped it by flexible pipeline to a tank on the shore, from which it was despatched to the plants in liquid form by custom-designed tankers.
Similar arrangements developed on the banks of the Humber, supplying the plants in that area, either with dug clay or with slurry.
Use of alluvium was problematic when rotary kilns began to be used, because of chloride cycles, and became fatal when electrostatic precipitators were installed, the chloride making the dust difficult to precipitate. All the south-eastern plants ended up using older bedrock clays.
Boulder Clays
Boulder Clays were produced as superficial deposits during the ice ages, and so are 0-2 million years old. Because they were formed by ice, they do not occur in the south of England and southern Ireland. The mechanism of formation involves a grinding action on rocks which gives rise to material of floury fineness in addition to larger fragments. The process involves intense weathering, so that clays deriving from source rocks that might be unsuitable (e.g. due to high sulfides or alkalis) are rendered innocuous.
Boulder clays were often used by the northern plants that used chalk ballast as their primary material, beginning with the first at Gateshead, although as far south as Masons, Boulder Clay overlying the chalk was used throughout the 20th century.
London Clay
London Clay is a sediment laid down during the Ypresian stage of the Lower Eocene, around 48-55 million years ago. The clay was laid down in tropical conditions, in a reducing environment, resulting in a blue colour due to the presence of pyrite. The clay oxidises on weathering, turning the colour yellow due to conversion of pyrite to hydrated iron oxides.
London Clay was familiar to early cement manufacturers because it was the matrix containing the original septaria used in making Roman Cement. It is a curious fact that the many plants that started by making Roman Cement, and graduated to making Portland cement, chose to use alluvium - often brought long distances - as their secondary raw material, although London Clay was close at hand. (Possible exceptions are Rainham and Upnor.) The use of London Clay began when the industry started to spread from its original home in the Thames and Medway estuaries. The first seems to have been Harefield (1880).
The initial objections to the use of London Clay (apart from mere superstition) were the fact that its fineness is variable, so that it often becomes sandy, and its sulfur content, which was largely retained in the clinker in batch kilns. The sand content required either fine screening of the slurry, or in more recent times, the use of re-grind ball mills.
The availability of the classic tidal alluvium started to be restricted during the twentieth century, and during the 1920s, as part of their expansion programme, most of the large Thames-side plants opened London Clay quarries, slurrying the clay at the quarry, and pumping it to the plant's main washplant for incorporation in the rawmix. This technique reached its zenith with the provision of the 3.5 million tonne a year Northfleet plant with clay from Ockendon (Essex), involving an 11 km pipeline under the Thames. This quarry dug, at peak, over 1.5 million tonnes of clay a year. The sulfur content of the clay continued to be a significant problem, and effectively prevented the wet process Northfleet plant from achieving slurry moisture contents below 38%.
With the extinction of the industry in the south-east, London Clay is no longer used in cement manufacture.
Gault Clay
The Gault Clay is the representative of the Albian Stage of the Lower Cretaceous (100-110 million years ago) in southern England, where is commonly forms a broad vale at the foot of the chalk escarpments. It represented a transitional stage during the onset of the conditions that resulted in the formation of the chalk, and because of this, it is calcareous, with the calcium carbonate content usually rising towards the top of the formation, and sometimes reaching 50%.
Due to its location, it was convenient for use by plants on the scarp face of the chalk, but in practice it was not used where the intervening Chalk Marl was available.
An early user was (probably) Aylesford (1874), located on a Gault quarry from which bricks were originally made. A similar story accounts for its use at Arlesey (1883). The Sussex dip-slope plants at Shoreham and Newhaven converted from using Medway alluvium to the more convenient Gault in 1897, and Rodmell and Westbury used it from the outset, the latter eventually abandoning it in favour of Jurassic clay. Apart from these, Gault was only used in a small way as an occasional corrective at Marl-based plants such as Rochester and Holborough and Dunstable.
The Gault (particularly the upper part) was fairly rich in swelling clays, so that its slurries were difficult to deflocculate.
Upper Jurassic Clays
The Upper Jurassic is divided into three ages:
- Tithonian (traditional British "Portlandian"): 145-152 million years old
- Kimmeridgian: 152-157 Ma
- Oxfordian: 157-163 Ma
Throughout England, the Kimmeridgian and Oxfordian form a broad flat vale of clays. The Oxfordian is locally divided into the Ampthill Clay above and the Oxford Clay below. They are usually highly carbonaceous (the Kimmeridge the most so) and can be quite difficult to distinguish from each other. In central England they once formed the world's largest brick-producing region. The clays are relatively low in silica, and a supplementary source of this (i.e. sand) is often necessary. In some places, these clays are separated from the toe of the chalk outcrop by only a narrow intervening sequence, so that they form convenient clay sources. Three significant examples are:
- Westbury, using Kimmeridge Clay
- South Ferriby, using Kimmeridge and Ampthill Clays
- Humber, using mainly Ampthill Clay
Middle Jurassic Clays
The British middle Jurassic is extremely varied in composition, with clays and sands occuring above and below the limestone beds, and so plants using Middle Jurassic limestone used whichever clay bed was most convenient.
At Kirtlington and Oxford the Forest Marble Formation was used. This is sandwiched between the Cornbrash Limestone above and the Great Oolite (White Limestone Formation) below, and in the locality varies laterally from clay to limestone.
At Premier, a low-grade Oolitic limestone was used alone, but in earlier times, some of the under lying Upper Lias clay ((Whitby Mudstone Formation) was added.
At Ketton, the main limestone used is the Inferior Oolite (Lincolnshire Limestone Formation), which is overlain with a clay (the Rutland Formation) at the base of the Upper Oolite.
Carboniferous Shales
The shales used in cement manufacture can conveniently be divided by geological stage:
- Westphalian (traditionally in Britain, "Coal Measures"): 304 to 315 million years old
- Namurian ("Millstone Grit"): 315-326 Ma
- Dinantian ("Mountain Limestone"): 326-359 Ma
Because for users of Carboniferous limestone, there were usually other shales nearer at hand, Westphalian shales were not much used, and their use was restricted to small early plants in the West Midlands, such as Deepfields, Oldbury and Windmill. A special case is Padeswood, which has throughout its life used colliery waste as its secondary raw material.
The Namurian often overlies Carboniferous Limestone used as a primary raw material, and in some places it is shaly. One example is Platin.
The majority of plants using carboniferous limestone use stone from the Visean (Upper Dinantian) Stage (331-347 Ma) and in many instances a shale formation lies immediately above the limestone, and is consequently used. Examples are: Ballyconnell, Cauldon, Cookstown, Derrylin and Hope. A special case is Tunstead, at which the otherwise ultra-pure limestone is interbedded with thin layers of shale ("wayboard") which was removed by selective crushing and washing in order to produce chemical-grade limestone. The cement plant was originally set up to absorb these "tailings". In other instances, and in particular in the older (Tournaisian) part of the Carboniferous, and in the northern parts of Britain, limestone and shale are intimately inter-bedded and can be treated as a single unit.
Pre-carboniferous Shales
The oldest shales known to have been used are from the Silurian. Where these shales are weathered (i.e. are low in alkalis and sulfur) and the silica content is fine, they can be good raw materials. Some plants using carboniferous limestone have good Silurian shales nearby, and the plants known to have used them are Drogheda, Ellesmere Port and Limerick.
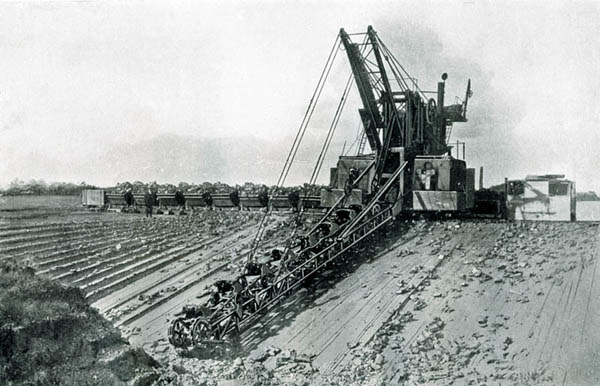
Very early (1913) multi-bucket excavator extracting alluvial clay at the Barrow on Humber clayfield supplying Wilmington. The machine discharges into tram-cars which are then hauled to the washmills on the quay. The clay was moved to the plant by barge in slurry form. Later forms of MBE discharged directly onto belt conveyors.