Kiln Exhaust Gases
The mass of exhaust gases that exit from the cold end of a rotary kiln exceeds the mass of clinker produced. For each tonne of clinker made, the gases consist broadly of:
- around 0.6 t of gases (mainly carbon dioxide) evolved by the raw materials.
- fuel combustion gases: around 1.1 t from a modern efficient kiln, but three or four times as much from early kilns.
- in wet process kilns, between 0.5 and 1.5 t of water vapour evaporated from slurry.
- varying amounts of air, deliberately added or the result of inleaks: 0.2 t at least.
Early static kilns, although the relative amount of exhaust gas was at the high end of the scale, produced clinker so slowly that exhaust gases were emitted at very low velocity. Rotary kilns transformed this situation, and for the first time produced high-velocity exhaust streams that carried a certain amount of dust.
The cement industry was brought within the scope of the Alkali Act in 1881 (Alkali, etc., Works Regulation Act, 1881), for the curious reason that the Act covers the manufacture of “aluminous materials”. Most Inspectors were already busy with serious polluters and confined themselves to occasional measurements of HCl and H2S which were the main pollutants at alkali works. The arrival of rotary kilns in the 1900s, with a chorus of dust complaints brought much closer attention. Rapid developments in the design of kilns initially kept the Inspectorate at bay, and the Chief Inspector saw the lengthening of kilns as a means of eliminating the problem.
The construction of the bottle kiln was largely replicated in the later forms of batch-process static kiln. The base and the conical section were built from ordinary bricks and mortar (although concrete was occasionally used). Iron hoops around the outside of both the base and the cone provided tensile strength and allowing thinner brickwork. The lining of the kiln was made with “fire-brick” – the typical siliceous refractory of the early industrial period. The lining was made smooth and to a carefully controlled curvature by building against standard wooden formers. A doorway was made in the cone to allow the feed materials to be put in. Sometimes a second door was also provided half-way up the kiln. At the base, a grate of wrought iron bars was provided, and an access tunnel led from this to the outside. The air supply for the kiln passed through this tunnel, and it was common to align the tunnel towards the prevailing wind to improve the draught. A small access door between the tunnel and the kiln immediately above the grate was used to extract the finished clinker. All the side doors were bricked up during burning, and plants maintained a team of bricklayers for this and for repairs to the kilns’ refractory linings.
Dust Emissions
The solids leaving a cement kiln system in suspension in the exhaust gases are called cement kiln dust (CKD). The clinker that is produced by cement kilns varies little in chemistry from one site or kiln to another, and it is a common fallacy to assume that CKD is similarly uniform. The nature of dust emitted is extremely sensitive to the nature of the raw materials and the type of production equipment, and may even vary considerably on a single kiln, using a single set of raw materials.
Dust is generated in a kiln system because, at various points, the process material is in a finely divided form, and is in contact with moving gases. The process whereby particles are picked up, and kept in suspension, by a gas stream can be understood in terms of a simplified model, in which the terminal velocity in air of spherical particles of density 2700 kg/m3 (typical of rawmix) is calculated.
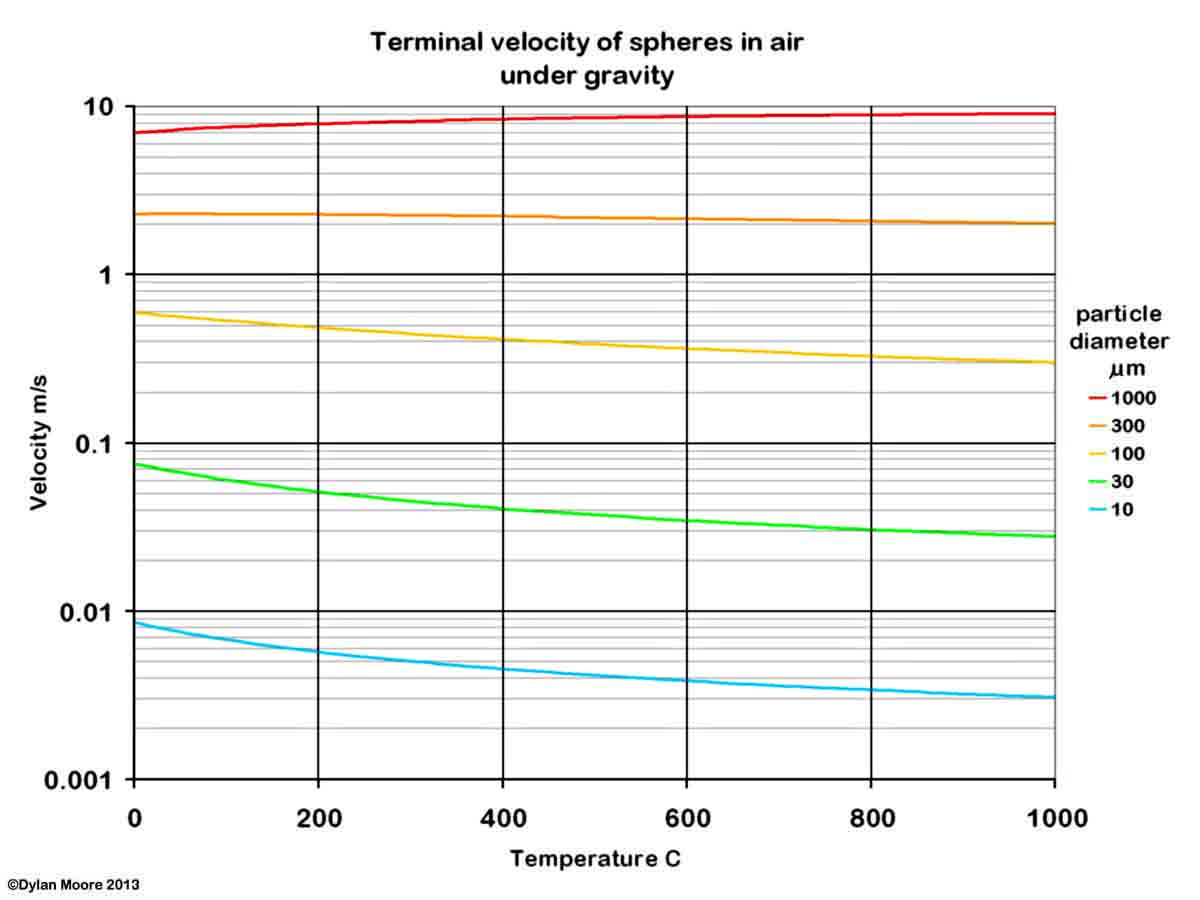
It will be seen that the finer particles of rawmix (< 30 μm) are easy to pick up, and are unlikely to settle out under the action of gravity alone. Separation of the dust from the gas stream can be achieved by applying a force greater than that of gravity. In a good cyclone, a centrifugal effect of 10g might be obtained, increasing the terminal velocity.
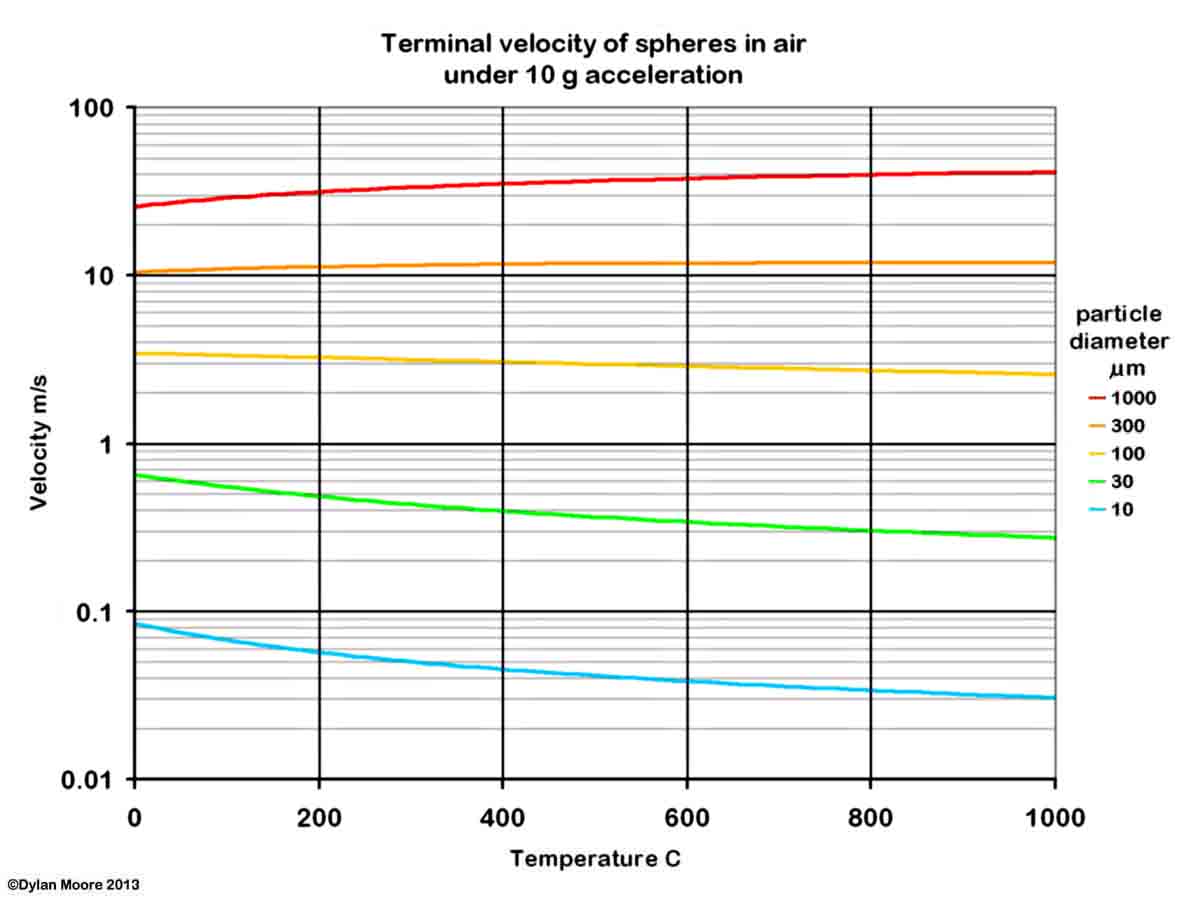
In a horizontal kiln with a counter-flowing gas stream, the amount and nature of dust picked up depends upon the feed particle size distribution and the composition of different size fractions. In a powdery feed, the harder minerals concentrate in the larger size ranges, while the softer minerals concentrate in the ultra-fines, and therefore also in the dust that is picked up. In addition to material picked up from the kiln feed, the solids carried in the gases contain a small, but very significant, amount of "fume" particles of diameter much less than 1 μm, produced by the condensation of salts that were evaporated in the kiln's burning zone.
The composition of the dust is of interest because some escapes as an atmospheric pollutant, and because much of the dust that is captured has historically been dumped (although modern landfill taxation is eliminating this practice). Naturally, the dust mainly consists of rawmix, comprising mainly calcium carbonate and clay minerals. A certain amount of calcined material may be present, but is not usually significant. Although small in quantity, condensed burning zone volatiles can constitute a major proportion of the dust when the dust loss itself is small. The amount of these depends mainly upon the amount of sulfate, and more particularly chloride, in the system. Chloride is evaporated in the burning zone as NaCl and KCl, and also encourages the volatilisation of elements - some of which are pollutants subject to special control - that have volatile chlorides. These include zinc, cadmium, mercury, thallium and lead.
Early Rotary Kilns
Early rotary kilns discharged exhaust gases into a back end chamber to which the stack was directly attached. Gases were drawn through the kiln solely by virtue of the convective draught produced by the stack. The convective effect results from the fact that the exhaust gases are less dense than air, and the amount of suction produced by the stack is proportional to the height of the stack and to the density difference between the gas and the ambient air. The main reason for the lower density of the exhaust gas is its high temperature. For this reason, in order to maximise the kiln output, it was necessary to maintain a high exhaust gas temperature, and this was a dis-incentive to improve energy economy. A 20 m kiln would not work with an exit gas temperature less than around 600°C, and for this reason, early stacks were usually made of steel.
An early argument against the lengthening of rotary kilns from the early 20 m or so was that the extra length required more suction, while the extra heat exchange provided by a longer kiln caused a reduction in exit gas temperature, and therefore a reduction in stack draught. The sales representative of Amme, Giesecke & Konegen, while quoting John Hudson Earle for cement plant, told him he "does not believe in 250ft kilns as not enough heat owing to length to get smoke up chimney - says 150ft will turn out as much as 250ft". Earles went on to buy long kilns from FLS.
These kilns soon became the focus of public attention because of the amount of dust and grit emitted, and methods of mitigating this began to be developed. On wet process kilns, the first attempt was the "wet bottom" back end chamber in which the kiln gas was drawn downwards over a reservoir of water or slurry before rising into the stack. The cross-sectional area of the duct was increased in order to slow down the gas, improving the chances of dust dropping out. In some designs, the chamber was very large, with a cross-sectional area as much as 20 times that of the kiln, but it will be appreciated from the above charts that this contributes only marginally to dust drop-out. Sometimes, paddles were fitted to lift the liquid into the gas stream. These devices would scrub out most of the coarser dust provided that the kiln draught (and therefore output) was kept low. A typical example installed with these features is Aberthaw kiln 1 and 2 (1914) where each 200 ft kiln was supplied with a 200 ft brick stack.
Induced Draught Fans
The inefficiency of using stack suction to provide kiln draught could be avoided by use of a fan to draw the gases out of the kiln. In the UK at least, this did not emerge as a technology until after WWI, perhaps because of the size of electric motor needed (when electricity was not necessarily available at all) and perhaps because of the engineering challenges posed by a large fan handling hot and abrasive (and perhaps acidic) gases. The first kilns installed with fans were on Humber kilns 1 and 2 (1921), of similar size to those at Aberthaw, for the curious reason that the terms of the land-lease restricted stacks to 75 ft in height, and these could not produce sufficient draught.
This term was easily re-negotiated after the surrounding country had been plastered with dust for a few years.
Apart from these, kilns continued to be installed without fans. However, the advent of chain systems (from 1929 onwards) as heat exchangers in wet process kilns brought about a restriction to gas flow that mandated the addition of fans. This corresponded with a dramatic increase in exhaust gas flow rates. The addition of a chain system increased kiln output by 30-50%, and exhaust gas velocity naturally increased pro-rata. This caused a further step-change in dust emissions. However, a much more significant contribution to dust pick-up was the grinding effect of the chains. In wet kilns with no heat exchangers, the dried material consisted of fairly large lumps, and fines that might be picked up as dust derived only from abrasion of the lumps, and a certain amount of decrepitation during de-carbonation. The addition of chains broke up the slabs of semi-dried material much more vigorously. Because the resistance of the material to breakage diminishes as the water evaporates, systems were designed to leave 1-5% moisture content in the material leaving the chains. While this might be achieved on average, variations in operating conditions led to exaggerated variations in the condition of the feed at the end of the chains, and consequent large oscillations in dust production.
It is often suggested that dust pick-up in a rotary kiln is proportional to gas velocity to the power n, where n is 3-6. This, of course, is nonsense. The rate of dust pick-up is controlled by the shape of the feed particle size distribution curve and the individual particle terminal velocity.
The concentrations of bigger kilns in the 1930s gave the cement industry a bad reputation for dust emission, with drifts of dust in the streets of the main industrial areas. The Alkali Inspectorate responded by putting pressure on the industry to clean up exhaust gas streams, but mainly by insisting on the construction of tall stacks. A rotary kiln with an induced draught fan doesn't really need a stack at all, but tall stacks from here on became a feature of the UK industry. These culminated in the 550 ft stacks at Northfleet. The underlying philosophy of the high stack is to spread the pollutant more thinly over a wider area.
The invention of preheaters for dry process kilns, because these always produced a gas pressure drop, also necessitated the use of fans, and in more complex designs, multiple fans may be used. Each cyclone stage in a suspension preheater involves a pressure drop that must be overcome by fan suction, and the main fan is easily the biggest drive on a modern kiln system.
Dust Control Equipment
Dust, purely because of its visibility, became the main focus of pollution controls by the Alkali Inspectorate. The early "wet bottom" technique, was ineffective with any but the most leisurely gas flow-rates. From the 1920s, the types of equipment still in use became available.
- cyclones
- electrostatic precipitators
- bag filters
All these are inherently less effective at collecting small particles than large particles, and the overall collection efficiency therefore depends upon the particle size distribution of the dust.
Cyclones work by applying a centrifugal force to the suspended particles. Collection of a particle depends upon the particle, under the influence of the force, being able to migrate to the edge of gas stream during the time that it remains in the cyclone. Smaller particles, with a lower terminal velocity, are therefore less easily captured. Cyclones, unlike the other types, become more efficient as the gas flowrate increases, due to the increased centrifugal effect. However, the pressure drop increases with the square of the flow-rate, so that the fan power required becomes enormous for even a moderate efficiency. The maximum efficiency practicable is about 95%, but in practice, efficiencies over 90% are hard to achieve. However, cyclones do have the advantage that their operation is independent of temperature and gas composition.
Electrostatic precipitators pass the gas through a "corona discharge" produced by pairs of electrodes producing an electric field of 200 kV/m or more. This faintly-glowing discharge causes the dust particles to gain electrons whereupon they are attracted to the positive electrode. On reaching the positive electrode, the excess electrons are discharged, and the particle sticks. Some sort of "rapper" occasionally shakes the accumulated dust off the electrode into a hopper at the bottom. As with the cyclone, the efficiency is limited by the terminal velocity of the particle, which must migrate across the gas stream during the brief period in which it passes through the field, so efficiency is lower for smaller particles. The power consumed is essentially that conducted through the gas by the corona discharge, and for a given power consumption, electrostatic precipitators are much more efficient than cyclones. A 90% efficiency can be obtained with minimal power at 30 kV, while, if the applied voltage is increased to 60 kV, efficiency of over 99.9% can be achieved with a typical cement kiln dust. To a certain degree, the efficiency increases with dust loading, as the larger charged particles can "frog-march" smaller uncharged particles out of the gas stream. Unlike the cyclone, the precipitator has very little pressure drop, so extra fan power is not needed. Because of this and its greater efficiency, the precipitator became the method of choice.
The precipitator dates back to Cottrell's patent of 1907, but the early designs were for the removal of liquid droplets from air, and their use for solids only began to develop in the 1920s. The first installation in the UK cement industry was at Wishaw in 1928. Two were retrofitted on Johnsons kilns 6 and 7 in 1933, although "mist precipitators" had been installed on the first Billingham sulfuric acid kiln (installed 1930) from the outset. The increased public visibility of the industry's dust emissions - exacerbated by the installation of chains in the 1930s - led the Alkali Inspectorate to decree in 1935 that all new kilns installations should have precipitators and maintain emissions below 0.5 grains per standard cubic foot of exhaust gas (1206 mg/m3 at NTP). However, as always, the application of these rules was "negotiable", and on older plants, new kilns continued to be installed without precipitators. However, precipitators were normally installed as standard on new plants after WWII, and progressively more sophisticated and efficient designs were employed.
As performance improved, with efficiency over 99% considered normal, the performance mandated by the Alkali Inspectorate also increased, until certain limitations of precipitators began to emerge.
Firstly, because the precipitator is an electrical device, an explosion can occur if the gas stream becomes explosive - as can occur in a cement kiln if incomplete combustion takes place and carbon monoxide is formed. For this reason, precipitators were designed to cut out if the gas carbon monoxide content increased above a certain level. On some kilns, "spikes" of carbon monoxide formation were common enough for the resulting precipitator outages to cause significant emissions.
Secondly, precipitator efficiency is affected not only by particle size, but also by the conductivity of the dust. The system only works well if the particles are sufficiently conductive to easily acquire and discharge electrons. Raw material and clinker particles are easily precipitated, but crystalline salts have high resistivity. Excess alkalis, sulfate and chloride are concentrated in the kiln dust because of their volatility. The dumping of the dust "bleeds" these elements out of the system. Problems became more severe when recycling of dust became more common: under these circumstances, alkalis, sulfate and chloride build up in the kiln system. Volatilised salt vapour, when cooled, crystallises out on the surface of dust particles and produces a resistive layer, so that precipitation efficiency diminishes. A certain amount of the vapour also crystallizes as individual crystalline "fume" particles only a few nanometres in diameter. These can't be precipitated, and because of their strong tendency to scatter light, have a high effective opacity. (Opacity is the normal method of assessing dust emission on modern systems).
Thirdly, although long wet process kilns produced gases that were wet and cool (around 200°C), the newer dry process kilns produced gas that was much drier and hotter. Precipitators do not perform well with hot, dry gases, and necessitated the addition of expensive and unreliable conditioning towers to wet the gas. For these reasons, precipitators are now less often regarded as the "best available technology".
Bag filters are just like giant vacuum cleaners. A large number of sock-like bags are arranged in an enclosure, inlet downwards, the gas is drawn through, and the bags are periodically beaten or subjected to reverse gas-flow in order to dislodge the dust into a hopper at the bottom. Clearly, by use of suitably finely-woven bags, any desired efficiency can be achieved, at the expense of a considerable pressure drop. The obvious reason that bag filters were not initially a viable option was the sensitivity of bag materials to high temperature. The use of bag filters on kilns developed in the USA, with gradual improvements in bag materials, particularly the use of PTFE and glass fabrics, and with fool-proof methods of controlling the gas temperature within the service range. It remains the fact that, whereas with a precipitator a sudden change in gas conditions may cause emissions to increase for a while, in the case of a bag filter, complete destruction of the filter may occur. However, bag filters are "fail-safe" in case of carbon monoxide spikes and alkali fume surges.
Notwithstanding these problems, bag filters are now often used on new installations, and several precipitator plants have been converted to bag filters.
Gaseous Emissions
Kiln exhaust gas contains various gaseous species that are regarded as pollutants:
- carbon dioxide
- carbon monoxide
- sulfur dioxide
- hydrogen sulfide
- nitric oxide
Carbon dioxide derives from decomposition of raw material carbonates and from combustion of carbon-containing fuels. Up to the present, the means of minimising it have consisted of lowering the amount of carbonates in the raw-mix (not a serious proposition if Portland clinker is to be made, except in the case of the Anhydrite process), by use of high-hydrogen fuels (i.e. natural gas, currently excessively expensive) and by reducing the fuel consumption. The 60% reduction in carbon dioxide generated per tonne of clinker that has taken place since 1895 has been entirely due to reduction in fuel consumption, although it can be argued that the recent use of "bio-mass" as a fuel is a "carbon-neutral" process. Carbon capture has been used in the past in the cement industry, and remains a future possibility.
Carbon monoxide is produced in a kiln if there is insufficient air for complete combustion of the fuel, or if the fuel doesn't spend enough time at combustion temperature for the combustion reaction to be completed. Carbon monoxide was produced in quantity in early rotary kilns due to their short length, and was often accompanied by black smoke produced by unburned fuel. Manufacturers were conscious of this as a loss of available energy, and as kilns became larger and gas analysis methods developed, care was taken to make CO generation a rare occurrence. Since it is accompanied by "reducing conditions" in the burning zone, causing very negative effects upon clinker quality, the avoidance of CO generation became rigorous. The first continuous carbon monoxide analyzers became available in the 1920s, and became standard in the instrumentation of every kiln, allowing the kiln operator to instantly respond to its formation by adjusting the fuel/air ratio. From the 1950s onward, fuel/air ratios have routinely been automatically controlled. In modern precalciner systems, the deliberate formation of CO at the preheater burner has been adopted as a means of removing NO in "staged combustion":
2NO + 2CO → N2 + 2CO2
This involves restricting tertiary air in-flow so that fuel is in excess. The remaining tertiary air is then added, allowing the remaining CO to burn out.
Sulfur dioxide derives from combustion of sulfur and sulfides in the fuel and the raw materials, and from the reductive decomposition of sulfates. Early rotary kilns retained little sulfur in the clinker, and most of the sulfur input ended up as sulfur dioxide. Particularly on wet process, raw materials high in sulfur were avoided, and the majority of the sulfur usually derived from the fuel. In this, the industry was no different from all the other coal-consuming industries. Modern kilns are capable of capturing a large proportion of the sulfur in the clinker, due to the scrubbing action of the fine calcium carbonate in the preheater, but nett inputs of sulfur have also increased. Concern with sulfur dioxide as a cause of "acid rain" has led to a number of high-emitter kilns being fitted with exhaust gas scrubbers. These react the gas with calcium carbonate or hydroxide slurries, producing calcium sulfite, which is gradually oxidised to sulfate, and can be used as a gypsum replacement. However, most kilns produce considerably less sulfur dioxide than was historically the case by careful combustion control. Sulfur dioxide is now routinely continuously measured, and increases in the level are indicative of the onset of burning zone reducing conditions, which is avoided.
Hydrogen sulfide historically was detectable in kiln exhaust gases whenever carbon monoxide was produced, due to the lack of sufficient air to burn the sulfur. Being one of the emissions controlled by the Alkali Inspectorate in the alkali industry, regular emitters were penalised. After WWI, its occurrence is a rarity, and is prevented as long as carbon monoxide formation is being actively avoided.
Nitric oxide is produced by two mechanisms: by oxidation of fuel nitrogen which normally occurs in the kiln main burner flame, and by thermal reaction of air nitrogen with oxygen in a hot burning zone. Nitric oxide, in addition to being an "acid rain" producer on oxidation to nitric acid, is also a cause of photochemical smog. The concentration of nitric oxide in cement kiln exhaust was never measured before the 1970s, but it is reasonable to suppose that it has always been a feature of rotary kilns, where a very hot flame is employed. Concern about its effects has led to measures being taken to reduce the amount emitted. This is not straightforward, since the flame temperature, which controls the amount of "thermal" NO produced, can't be reduced much without drastically reducing the output of the kiln. A number of approaches have been employed:
- the use of "low-NOx" burners which produce reducing conditions in the hottest part of the flame so that NO formation is discouraged, and excess air for hydrocarbon and CO burn-out is only introduced in the cooler part of the flame.
- the use of "staged combustion" (see CO above) in a precalciner: an initial excess of fuel causes formation of carbon monoxide which reduces the main burner NO to nitrogen, then tertiary air is added to burn out the excess carbon monoxide.
- the use of ammonia injection: ammonia reacts with NO at moderate temperatures to produce nitrogen and water.
6NO + 4NH3 → 5N2 + 6H2O
Kiln exhaust NO is now routinely continuously measured, and the above mitigation measures make use of the data in automatic control systems. The NO value is also used as a sensitive indication of burning zone temperature.