The following is a transcript of an anonymous article that appeared in The Engineer, 54, 11/8/1882, p 98, together with an extract from Redgrave's Calcareous Cements: their nature and uses, both of which are believed to be out of copyright. The Folkestone plant is not included among the plant accounts given in this site, because it closed (1891) before the commencement date (1895) of this study. However, this article is presented here because it gives an unusually clear account of a plant in the pre-scientific era, of an early excursion outside the Thames/Medway area, and because it is clearly an early work of Charles Spackman, who was later a prolific writer on cement technology and history. Note on Imperial units of the time: 1 ton = 1.016047 tonnes: 1 ft = 0.304799 m: 1 in = 25.4 mm: 1 h.p. = 0.7457 kW: 145.037 psi = 1 MPa.
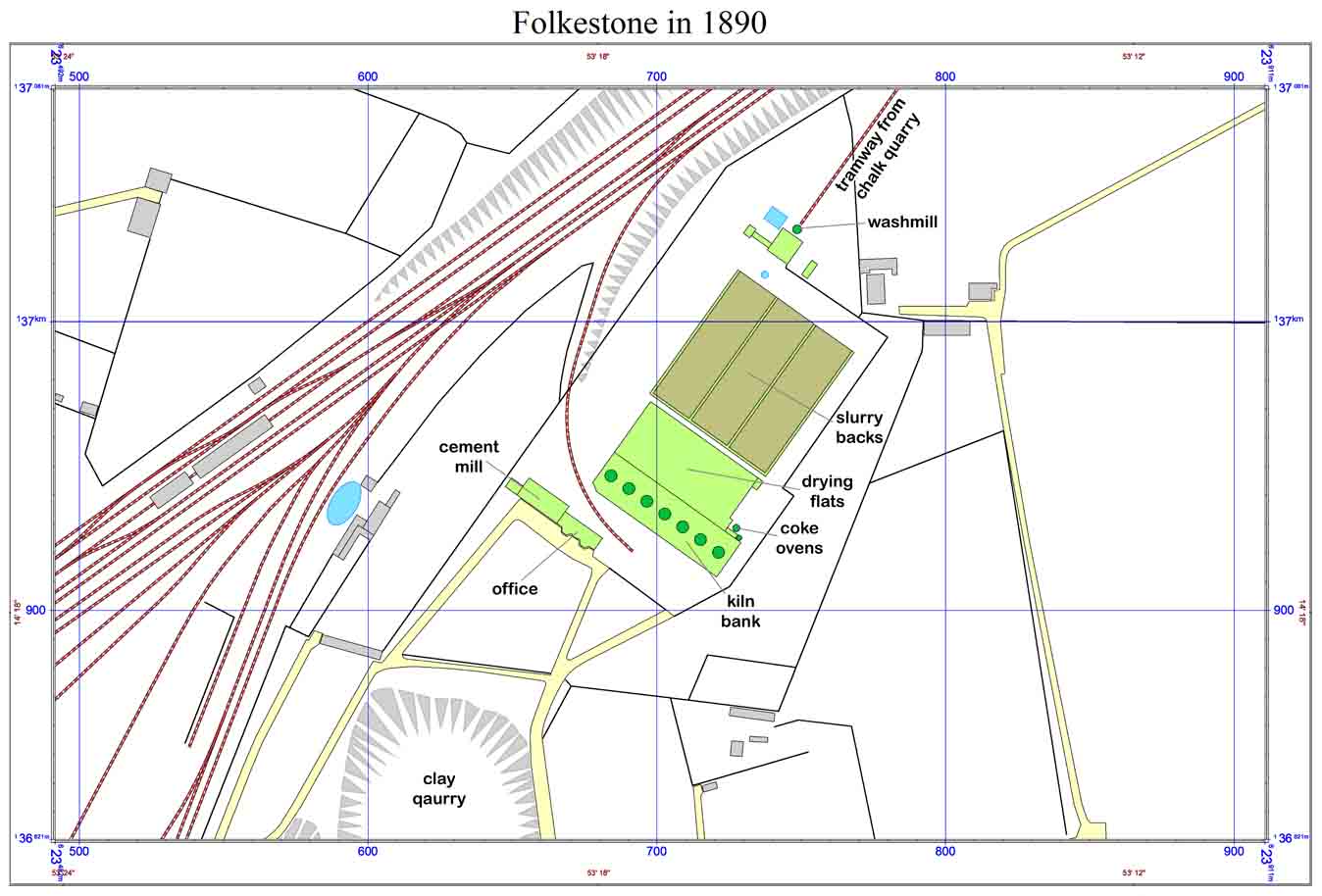 Location of the cement plant. The railway was constructed in 1843-4, and a coal depot - initially with a coke plant - was located at the junction with the harbour branch.
Location of the cement plant. The railway was constructed in 1843-4, and a coal depot - initially with a coke plant - was located at the junction with the harbour branch.
THE MANUFACTURE OF CEMENT AT FOLKESTONE.
In the new harbour works of the South-Eastern Railway Company, now in course of execution at Folkestone, artificial blocks, made of pebbles from the sea beach, bound together with Portland cement, are largely used. The Portland cement is obtained from the Folkestone Cement Works, about half a mile from the harbour (Note 1); and at the cement works the raw materials are obtained close at hand. The works stand upon an inclined plane; chalk is brought to them from the cliffs above, and clay is wound up to them by a stationary engine from a clay-pit a hundred or two yards from the place of manipulation. A correspondent, who recently visited the works, sends us the following: -
The Folkestone Cement Works were established and carried on for two or three years as a private speculation (Note 2); in 1873 they were bought up by a company consisting chiefly of residents in the neighbourhood; at the present time Mr. John Minter, solicitor, is the chairman of the company; Mr. J. B. Judge, secretary; and Mr. Charles Spackman (Note 3), manager.
The materials for the manufacture of Portland cement vary in different districts. On the Thames, white chalk and alluvial mud found in the marshes adjoining the Medway are used; on the Medway, grey chalk and the same mud; in the Lias districts, the limestone shales and clays of the deposits furnish the most suitable materials. At Folkestone grey chalk and the gault clay which underlies the grey chalk, as already stated, are the raw materials (Note 4).
At Folkestone the different beds of both substances vary considerably in composition. When free from water the chalk contains from 84 to 95 per cent. of carbonate of lime, the rest being clay; and the clay, in a dry condition, contains from 10 to 30 per cent. of carbonate of lime. The chalk upon an average contains 20 per cent. of water, the clay 25 per cent. The first object to be attained is to reduce these substances to a fine state of division, and at the same time intimately mix them together in such proportions that when deprived of water the mixture shall contain from 75 to 76 per cent. of carbonate of lime. This is done by what is known as the wet process. The raw materials are fed in measured proportions into a wash-mill, which is a circular trough in which a number of harrows revolve, driven by a steam engine of 14 horse power. A stream of water is constantly flowing in, and the action of the harrows, aided by the attrition of the particles against each other, reduces the whole to the state of " slurry " or slip, which contains from 60 to 70 per cent. of water; in this state it flows into the settling tanks or "backs", each of which contains sufficient slurry to make 800 tons of cement (Note 5). Here it is allowed to subside, the supernatant water being drawn off from time to time. One settling tank is filled at a time, and then left for six or seven weeks for the solid matter to settle, the surface water meanwhile being drawn off from time to time by a small sluice (Note 6). The bottoms of the tanks are of the natural porous earth of the locality, and help a little to drain off the water by absorption.
To return to the wash-mill. No coarse particles of chalk must be allowed to pass into the backs, as their presence would endanger the cement. To guard against this the slurry is caused to flow away from the wash-mill through strainers of fine copper wire gauze. Catch-pits are placed between the strainers and the backs to intercept any coarse particles that may have escaped. The process is a continuous one, a stream of water with successive charges of chalk and clay constantly going in, and a stream of slurry continually flowing out. The rate of flow has to be carefully regulated, so as to secure a proper mixture. Some of the beds of clay are easily washed down, while others are most tenacious and difficult to break up. Some of the chalk is soft and is easily washed; other beds, notably that called the "burr chalk" - which is a kind of junction bed between the upper and lower chalk deposits (Note 7) - are harsh and gritty in the breaking up. The mechanical condition of the slurry is continually examined during the process of washing, by washing a small known quantity through a fine sieve, and drying and weighing the residue. The composition of the raw materials being known, their accurate mixture in the required proportion is simply a matter of calculation (Note 8). To guard, however, against possible error, the percentage of carbonate of lime in the slurry is constantly determined. If the percentage is not correct the proportions are altered, while occasional stirring of the backs ensures a uniform product. Many methods of analysis may be adopted (Note 9). The lime may be determined directly, but the operation involves much time and tedious work, especially where, as in a cement works, many determinations have to be made. For all practical purposes a determination of carbonic acid in a dried and gently ignited sample of the slurry is sufficient. From this the percentage of carbonate of lime can be readily calculated. A form of apparatus which will give the required result in from fifteen to twenty minutes, and involves only one weighing, viz., that of the quantity taken for the determination, is that of Dr. Scheibler (Note 10). The carbonic acid is collected over water, its volume read off, and the weight calculated with the usual corrections for temperature and atmospheric pressure. Another method, which is very accurate, is to absorb the carbonic acid in a tube filled with potash, pumice, or soda lime, the tube being weighed before and after the operation. There are several forms of apparatus by which carbonic acid in determined as loss, that known as Parnell's being one of the most useful. When sufficiently stiff to be dug out, the slurry, still containing from 40 to 50 per cent. of water, is removed from the backs in wagons to the drying floor. This is heated by the waste gases from a range of coke ovens, in which just sufficient coal is coked to dry sufficient slurry in twenty-hours to load one kiln. The kilns are seven in number, from each of which 18 tons of cement clinker are drawn (Note 11). The process of burning is an intermittent one. The kiln is charged with alternate layers of coke and dry slurry, lit at the bottom by means of thirty baskets of coke laid upon brushwood faggots. These kilns are subject to great wear-and-tear, which are materially lessened by coating their sides with slurry before each charging. A charge is usually burnt off in thirty-six hours, after which it is allowed one or two days to cool. The charge is then drawn (Note 12). The fuel and slurry are distributed in the kiln according to the judgment of the burner. At these works one ton of cement is burnt with 20 bushels of coke (Note 13).
It may be mentioned here that slurry prepared with a somewhat lower percentage of carbonate of lime than that previously mentioned, say from 72 to 73 per cent., would require less fuel to burn it, and the resulting clinker would be more easily ground, but the cement would have a low tensile strength (Note 14). On the other hand, a higher percentage would largely increase the quantity of fuel required, give a dense hard clinker very difficult to grind, and the cement would be liable to crack and fly when used. The product from the kilns is a hard blue-grey clinker, from which all underburnt portions are carefully picked out. The clinker is crushed by one of Hall's multiple action stonebreakers, taken by an elevator to a chamber above the mill, from whence it descends to the millstones. After being finely ground it is spread out in the warehouse for a few days, when it is packed in casks or sacks for delivery.
Portland cement is usually tested as to its tensile strength, and every English engineer who buys it applies his own tests, instead of adopting one general and fixed rule, as in Germany (Note 15). At Folkestone the manager tests what strain the cement will bear after being kept for seven days under water. The test demanded by Mr. Brady at the Folkestone new harbour works is that it shall bear a strain of 810 lb. on a sectional area of 2¼ square inches seven days after moulding (Note 16).
During the process of grinding samples are continually taken for the purpose of being tested. Mr. Spackman informs us that during the year 1881 the average of the tests gave a tensile strength of 497 lb. on the square inch of sectional area (Note 17).
The total output of Portland cement at the Folkestone works is 120 tons per week.
Analysis of a Sample of Slurry taken from the Backs in April, 1882. (Note 18)
The slurry dried at 100 deg. Cent. contained:-
| Insoluble in hydrochloric acid: | |
| Silica | 14.956 |
| Ferric oxide | 1.943 |
| Alumina | 5.167 |
| Lime | 0.173 |
| Water of combination and organic matter | 1.203 |
| Total Insoluble | 23.442 |
| Soluble in hydrochloric acid: | |
| Silica | 0.230 |
| Ferric oxide | 0.493 |
| Alumina | 0.230 |
| Carbonate of lime | 75.357 |
| Magnesia | 0.201 |
| Sulphuric anhydride | 0.057 |
| Potash | 0.070 |
| Soda | 0.127 |
| Total Soluble | 76.765 |
| Overall Total | 100.207 |
Analysis of some Samples of Portland Cement.
- The Folkestone Cement Company's, March, 1880.
- ditto - September, 1881.
- From a works on the Thames, 1881.
- A sample which possessed a strength of 500 lb. on the square inch, made from a mixture of blue lias limestones (Note 19).
- is especially interesting. The cement was made by Mr. Spackman from refuse from the Channel tunnel boring at the end of 1881 (Note 20), and possessed a high tensile strength.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Insoluble residue | 1.260 | 2.566 | 2.894 | 4.909 | 1.674 |
| Silica | 20.990 | 18.917 | 21.307 | 18.583 | 23.832 |
| Alumina | 8.869 | 8.763 | 6.593 | 7.226 | 6.058 |
| Ferric Oxide | 4.998 | 4.412 | 5.386 | 5.108 | 3.127 |
| Lime | 61.351 | 62.472 | 61.459 | 61.040 | 63.129 |
| Magnesia | 0.669 | 0.841 | 0.449 | 1.565 | 1.206 |
| Sulphuric anhydride | 0.886 | 0.929 | 1.422 | 0.763 | 0.398 |
| Potash | 0.978 | 1.100 | 0.437 | 0.754 | - |
| Soda | - | - | 0.429 | 0.271 | - |
| Total | 100.000 | 100.000 | 100.376 | 100.219 | 99.424 |
In his 1895 book (page 81), Spackman's long-time colleague G R Redgrave gave some data that Spackman had gathered while at Folkestone. I have converted archaic units to (SI) where necessary.
Fuel used in Cement Burning. It is almost impossible to lay down any exact rules for the time occupied in cement burning, and the amount of fuel employed varies also considerably. The results with some carefully-weighed kiln charges, burnt in the ordinary way with inter-stratified gas coke at the Folkestone Cement Works, as communicated to us by Mr. Charles Spackman, F.C.S., were as follows:-
The slurry, which was specially well dried for these trials, was found on the mean of several determinations to contain 2 per cent. of water only. The coke was also fairly dry. Pieces of moderate size were used, except in the bottoms, for which larger lumps were picked out. Five brushwood faggots were used in each case to start the fires. In kilns Nos. 3 and 7 all the materials were accurately weighed in and the product was weighed out.
The weight of coke was in No. 3 kiln:-
| Tons | cwts. | qrs. | lbs. | (kg) | |
|---|---|---|---|---|---|
| In bottom on faggots | 0 | 7 | 1 | 24 | 379 |
| In layers interstratified | 7 | 4 | 0 | 20 | 7325 |
| On top of kiln | 0 | 7 | 0 | 0 | 356 |
| Total | 7 | 18 | 2 | 16 | 8059 |
The kiln yielded 19 tons 2 cwts. 2 qrs. (19432 kg) of good clinker and 6 cwt. 3 qrs. (343 kg) of yellow, and, therefore, to produce 1 ton of clinker the coke required was 8 cwts. 1 qr. 5 lbs (i.e. 41.48% on clinker). As the mean of repeated observations, 50 bushels of gas coke fresh from the works weigh 21 cwts., and therefore, 8 cwts. 1 qr. 5 lbs. are equal to approximately 19¾ bushels. The coke cost 2½d. per bushel, and, therefore, the cost of coke for burning 1 ton of cement was almost exactly 4s. (Note 21)
In No. 7 kiln, with the same careful observations, 7 tons 17 cwts. 2 qrs. 16 lbs. (8009 kg) of coke produced 19 tons 7 cwts. 2 qrs. (19686 kg) of clinker and 2 cwts. 1 qr. 12 lbs. (120 kg) of yellow - the coke used per ton of clinker was thus 8 cwts. 15 lbs. (40.68% on clinker); say 19.36 bushels, costing about 4s. as before.
In a third trial, the weights in which were arrived at by an average, each ton of clinker needed 18.66 bushels of coke, or say 3s. 11d. per ton for the fuel for burning.
NOTES
Note 1. The plant was short-lived and is not explicitly shown on any map, but the 1898 OS map shows what is clearly the plant, with the kilns at (TR) (6)23703,(1)36936, oddly labelled "Canning Manufactory". The zigzag tramway from the quarry, washmills, three slurry backs, drying flats and kiln bank are visible.
Note 2. A. J. Francis (p. 174) says the plant "was erected by a local builder, John Pope, in 1870". References in the Folkestone Express, Sandgate, Shorncliffe & Hythe Advertiser suggest that it was incomplete, but the engine, needed for finish grinding, was "officially started" on 20/6/1872. On 5th October 1872 the Folkestone Cement Company Ltd was launched, with a capital of £30,000 in £10 shares, under new management. Share number 1 was issued to James Thyer on 9/10/1872. From 1879 it had Charles Spackman as chemist, later manager.
Note 3. see Charles Spackman's biography.
Note 4. The chalk quarry is still visible at 624120,137470 on the collapsed edge of the Warren landslip. The clayfield, around 623620,136810 is now occupied by Thanet Gardens and the industrial estate. The chalk quarry face exposes the upper part of the Grey Chalk (modern designation Zig-zag Chalk Formation) with the Plenus Marls and Melbourne Rock (modern Holywell Nodular Chalk Formation) at the highest point. The Gault Clay quarry was mainly the upper part of the Lower Gault, with Upper Gault exposed at the highest point. The Gault is always calcareous, the Lower Gault less so, and calcium carbonate content was probably typically in the lower half of the 10-30% range later mentioned. The intervening Chalk Marl (modern West Melbury Marly Chalk Formation) was not used, although entirely suitable. The physical and chemical properties of these materials were intensively investigated during the geotechnical research for the Channel Tunnel project. They were absolutely typical of the corresponding materials used by the cement industry elsewhere.
Note 5. The map suggests that the slurry backs were 170' long and 50' wide. 800 tons of cement would need 98,100 cubic feet of slurry at 65% water content, so the backs would have to be 12' deep, which is not unreasonable.
Note 6. Reduction of water content from 65% to 40% represents a 53% reduction in volume. By continued settlement, it would have taken 20 weeks to get down to 20% water. This represents the economic decision that had to be made in the "wet" process: use (as here) upwards of 3 MJ/kg clinker extra energy in the drying flats, or install three times as much space for settlement.
Note 7. Called "curly burr" in the Medway Valley, this is the local manifestation of the Melbourne Rock, which is the hard band defining the base of the Middle Chalk.
Note 8. This is an odd statement, since he has already mentioned that both the chalk and the clay are variable, so the composition of the raw materials is by no means known. The "constantly determined" chemical analysis was done to compensate for the day-to-day variations.
Note 9. By this stage in his career, Spackman already had an encyclopaedic knowledge of analysis methods - an expertise that most plants lacked in 1882.
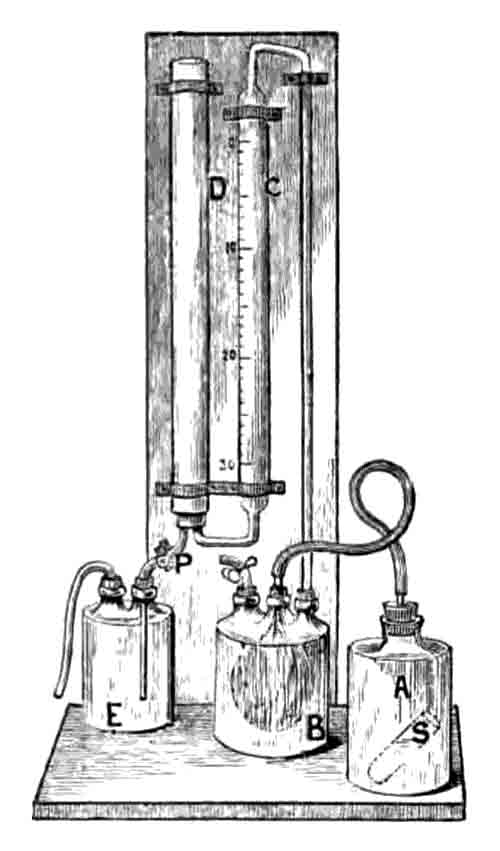 Scheibler's Calcimeter. The bladder B was a later addition to isolate the manometer water from the carbon dioxide. More complex and precise calcimeters were devised before the end of the century.
Scheibler's Calcimeter. The bladder B was a later addition to isolate the manometer water from the carbon dioxide. More complex and precise calcimeters were devised before the end of the century.
Note 11. 18 tons is the typical yield of a kiln of 13'6" (4.11 m) internal diameter. The map implies the kiln bank was about 180 ft long so the kilns were about 25 ft apart.
Note 12. The plant output of 120 tons/week implies that the total time for loading, burning and unloading was at least 7 days.
Note 13. At 0.021 tons per bushel, this represents 0.42 tons of coke per ton of clinker. If the coke had a calorific value of 31 MJ/kg, this means the burning energy was 13.0 MJ/kg clinker, which is at the low end of the range for intermittent kilns, but the rawmix was probably comparatively easy-burning.
Note 14. The trade-off between product quality and fuel consumption has been understood ever since Joseph Aspdin threw out his son for using too much fuel!
Note 15. The first German standard for Portland cement was issued in 1878. It was not until December 1904 that the first British Standard - BS 12 - was issued.
Note 16. i.e. 360 psi = 2.48 MPa. Assuming that this is a neat cement test, it corresponds to a modern EN 196 compressive strength of 2.3 MPa.
Note 17. 497 psi = 3.43 MPa. Corresponding modern EN 196 compressive strength 5.2 MPa.
Note 18. One can easily pick holes in these analyses, but they were as good as any at the time.
Note 19. Could be Barrow or Harbury.
Note 20. During 1881-1882, 7 ft pilot tunnels were bored about a mile from either end before the project was called off. As with the current tunnel, they were bored through the Lower Chalk. Each would have yielded about 12,000 tonnes of chalk - enough to keep the Folkestone plant going for more than a year.
Note 21. One might suggest that innovation was seriously impeded by having to struggle with a daft system of units. I'm alright - I've got Excel!