Louis Hengist Orsat was born 14/8/1837 in Paris. His father was a chemical manufacturer, and made blanc de céruse (PbCO3.Pb(OH)2) at Clichy. Louis entered the École Polytechnique, graduating in 1855. He became an engineer working on his own account, and became involved in steam raising and engines. He became aware of the efficiency implications of the fuel/air ratio, excess air resulting in waste of heat in hot exhaust gases, and excess fuel resulting in formation of carbon monoxide, with loss of the fuel's potential heat. He developed the apparatus now known as the Orsat Apparatus for analysing exhaust gases. He obtained a British Patent (No. 1853) on 22/5/1873.
He gave a long description of its application to optimisation of energy efficiency in the Annales des Mines (1875, 8, pp 485-506). His list of applications included furnaces, gasifiers, locomotives, blastfurnaces, reverberatory furnaces, lead chamber gases, CO2 generators, as well as respirable air. He went on to give case studies of several of these, including a long series of tests on locomotives in motion, on various French railways. The key attributes of the apparatus were its simplicity of operation "by an intelligent worker", and its compactness and portability, allowing it to be operated on a running locomotive footplate.
He was made Chevalier de la Légion d'honneur (décret 642 7/1/1871) for service in the Franco-Prussian War. He died in Paris 3/3/1882.
A funny thing: it was a lot more difficult than usual to find out about this guy. There were Wikipedia articles on the apparatus (needless to say, not very good) in English, German, Greek and Polish. On Orsat himself, nothing. And nothing in French!. Who would have thought it possible? I might just write a dummy French Wikipedia article suggesting (in view of his odd second name) that he was English. See if anyone bites (Note 1).
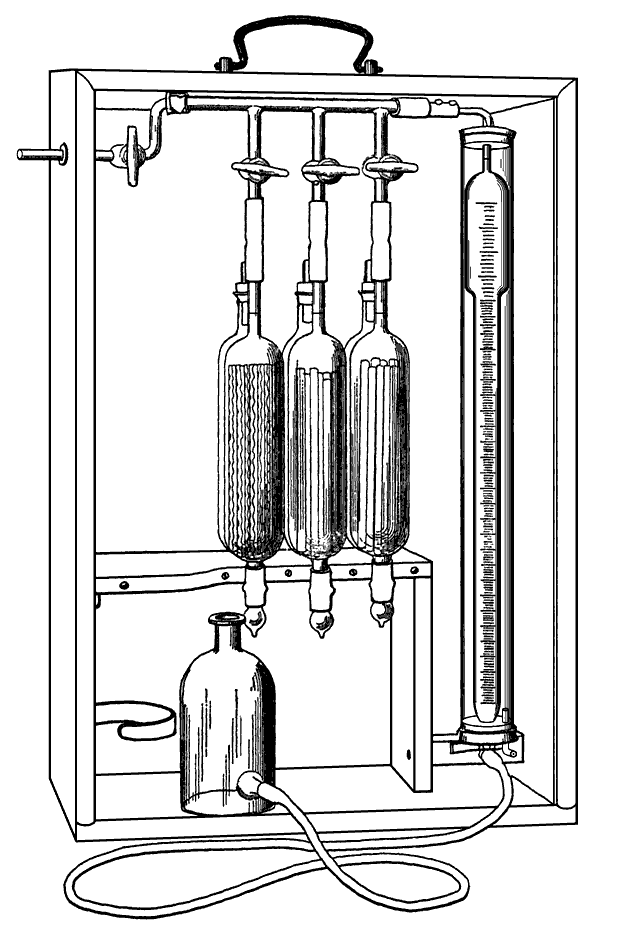
The Orsat Gas Analysis Apparatus most commonly used in the cement industry is shown right. For routine analysis of kiln exhaust gases, the sample was usually taken by drawing the gas into a water aspirator. This had the advantages that is provided a steady slow sampling rate over a long period (often several hours) and that it cooled the sample, condensing water, and washed out any dust.
The apparatus consists of a gas burette and three absorption pipettes, connected by a manifold with stopcocks. The burette (of capacity 100 mL and graduated with 0.1 mL divisions) is attached to a reservoir containing water, kept slightly acidic and coloured with methyl orange. Lowering the reservoir puts the burette under suction, drawing in gas, and raising it puts the burette under pressure, expelling the gas. The pipettes are large enough to accommodate 100 mL of gas, and are filled with absorbent solutions for (in sequence) carbon dioxide, oxygen and carbon monoxide. Many designs have been used, in order to speed up the sluggish absorption reactions, but typically the pipettes are packed with thin glass tubes to increase the wet surface area.
The first (right) pipette contains 55% w/w potassium hydroxide in water. This absorbs carbon dioxide, but also other acidic gases such as sulfur dioxide and hydrogen sulfide.
2KOH + CO2 → K2CO3 + H2O
The second (centre) pipette contains an alkaline solution of 1,2,3-trihydroxybenzene (pyrogallol) (Note 2). 50 g of pyrogallol is dissolved in 100 mL of water, and 900 g KOH is dissolved in 640 mL water, then the two solutions are mixed, preferably under nitrogen. The solution reacts with oxygen: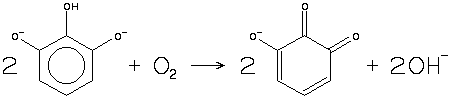
Because the solution is highly alkaline, it can also absorb carbon dioxide, so any carbon dioxide not absorbed in the first pipette will register in the second. If the solution has been used for some time, and the concentration of the product quinone has increased, secondary reactions occur, leading to the formation of carbon monoxide.
The third (left) pipette contains a solution of copper (I) chloride. A solution in hydrochloric acid is sometimes used, but the preferred option was ammoniacal, resulting in a complex that reacts with carbon monoxide:
[Cu(NH3)2]Cl + CO + 2H2O → CuCl.CO.2H2O + 2NH3
The pipette in this case is packed with spirals of copper wire, to keep the solution in a reduced condition and extend its life. Once again, this solution is capable of absorbing both carbon dioxide and oxygen, so any of these gases not completely absorbed in the previous two pipettes will register here.
It is a sign of the times that it is necessary to state what in the past would have been obvious - that for the preparation of these solutions, one needs to employ a professional chemist.
The test procedure is as follows:
The solution in each of the three pipettes is adjusted to the index mark on the inlet capillary.
Gas is drawn in from the aspirator by lowering the burette reservoir. Typically this is expelled and the burette is refilled to flush out any previous sample in the manifold. The volume of the sample is adjusted to 100 mL by bringing the level to the 0 mL graduation. All readings are taken with the reservoir at the same level as the liquid in the burette, ensuring that the sample is at atmospheric pressure. Once this has been established, the sample entry stopcock is closed.
The reservoir is raised to put the sample under pressure, and the stopcock of the first pipette is opened, allowing the gas sample into the pipette. The level is cautiously adjusted until the gas is entirely transferred and the liquid reaches the top index mark of the burette. Absorption is next allowed to take place. Standard methods suggest waiting for as much as three minutes, but it is common to "breath" the sample in and out of the pipette several times, since this increases wetting of the surfaces and turbulence. The sample is then drawn back into the burette, with great caution to ensure that the absorbent solution does not overshoot its top index mark, and the stopcock is closed. The reservoir is then brought level with the liquid in the burette, and a reading is taken.
The absorption routine is then repeated, until two consecutive readings are identical. If more than three cycles are needed, then the absorbant needs replacing. The reduction in volume is the dry-basis carbon dioxide content by volume.
The procedure is then repeated for the second and third pipettes, giving the oxygen and carbon monoxide contents.
An expert operator, working with care, can complete the entire test in about 30 minutes. During the period 1920-1970, it was normal on Blue Circle plants to analyse average exhaust gas samples once or twice a shift. On a four-kiln plant, this would take up 25-50% of a shift worker's time. Every plant had a "gas laboratory" next to the kiln exhaust ducts, with sample line plumbed in. Before 1950, the Orsat data was often the only data on combustion efficiency available for kiln operation. Larger plants installed continuous instrumentation for carbon monoxide from the mid-1920s onwards, and the installation of electrostatic precipitators (for which carbon monoxide formation must be eliminated) encouraged this. Continuous measurement of oxygen took much longer to develop, but by 1960, all plants had installed it. The Orsat apparatus remained in use to measure carbon dioxide - a not very informative quantity.
There are numerous sources of error in Orsat analyses:
- The effects of cutting corners are mentioned above: attempts to speed up the test lead to biases on oxygen and carbon dioxide.
- The although it is possible to correct for the dead volume of the manifold, this was rarely if ever done.
- There is a small effect from off-calibration burettes and difficult readability.
Detailed objective precision studies have been done, and 1-s values are of the order of 0.2 on carbon dioxide, 0.25 on oxygen and 0.3 on carbon monoxide (the latter two also subject to positive bias), so it's clear that its use was only justified for carbon monoxide levels more than 1% - a condition rarely encountered nowadays. Historical reported values for Orsat carbon monoxide below 0.3% should, by Occam's razor, have been reported as zero.
A further difficulty is the contact of the absorption solutions with the atmosphere in the rear limb of the pipette. It is possible, with some difficulty, to couple these to a reservoir of nitrogen to reduce this effect.
Notwithstanding these difficulties, a well-supplied and maintained gas laboratory running round the clock could provide valuable, reliable data. On the other hand, the use of the apparatus for occasional, ad hoc tests is never justifiable, because of the excessive amount of work for setting up and making fresh solutions, for the sake of a result of modest accuracy. Furthermore, on a modern plant with good instrumental measurements of other parameters, it is always more accurate to estimate the gas carbon dioxide content from first principles than to measure it by Orsat apparatus.
Today, cheap, fast, accurate hand-held digital oxygen meters are available at less than the cost a a fully-equipped Orsat apparatus, so use of the latter for oxygen is pointless, as is its use for trace-level carbon monoxide,