The following is a transcript of an article that appeared in The Engineer, 63, 4 March 1887, p 167. It is believed to be out of copyright. This was an important article, because of its influence on the subsequent development of the cement industry. I have resisted transcribing it until now (January 2016) because I felt that, far from adding to the knowledge of the subject, it rather subtracted from it. However, if read in conjunction with the notes, it will explain a key turning point in the history of the industry.
The article's author is named - not usual in The Engineer. Richard John Friswell (24/2/1849-6/2/1908) was a chemist, Director of the Atlas Aniline Dye Works, a leader in the setting-up of the Institute of Chemistry, and a founder member of the Society of Chemical Industry.
In the article, he develops the argument for the use of the rotary kiln - as designed by Ransome - in the cement industry. Up to this point, experiments with rotary kilns had taken the form of un-publicised exploratory work, undertaken at obscure sites (Mitcheldean and Barnstone) that were at the time new entrants to the industry. Ransome now installed a new, improved design at Gibb's Works, a plant in the heartland of the cement industry, and chose to attract publicity, which took the form of this article.
In order to emphasise the merits of the new system, Friswell first describes the classical, bottle kiln system of manufacture, listing its inefficiencies in detail, and in particular those of a discontinuous process. He then shows how these many inefficiencies are reduced or circumvented by a continuous rotary kiln. In the light of modern knowledge, most of the arguments presented are at least qualitatively reasonable, and are empirically confirmed by the universal use of the rotary kiln today.
However, unfortunately Friswell's plant visit and description jumped the gun: the plant he described was not operational, and was eventually abandoned as a total failure. A. J. Francis, in referring to the article says: "No defects are mentioned and one must assume that it was written before the trials had begun". This is probably a little unfair, since one can identify in the description some very clear defects, and so the kiln had obviously been run. It is particularly clear that it was producing under-burned material, and later that year, Ransome issued a patent amendment saying that a harder-burned product was preferred to that originally claimed. Friswell, as an outsider, had no way of identifying these problems.
Although the experiment failed utterly, no correction was ever made to this up-beat account, and it remained in circulation as a powerful recommendation of the rotary system. The degree of failure, here and at other places, and well-known by word of mouth, put the British industry off the idea of the rotary kiln for a decade. But the Navarro brothers, then commencing Portland cement manufacture in Pennsylvania, trusted the article as-read and proceeded to install a kiln exactly to Ransome specifications. They overcame the critical under-burning problem by the simple expedient of substituting the useless producer gas fuel with oil, and set in motion a rapid technological evolution, so that when British manufacturers finally decided to adopt the rotary kiln from 1900 onwards, it was in all its practical details an American invention.
Note on Imperial units of the time: 1 ton = 1.016047 tonnes: 1 cwt = 50.802 kg: 1 ft = 0.304799 m.
ON RANSOME'S IMPROVEMENTS IN THE MANUFACTURE OF PORTLAND CEMENT. By R. J. FRISWELL, F.I.C., F.C.S.
Portland cement is composed of lime, silica, and alumina in proportions approaching 60, 23, and 8 respectively; the remaining 9 per cent. consisting of various non-essential substances, such as ferric oxide, 3 to 5 per cent.; calcium sulphate, 1.0 to 3 per cent.; clay and sand, 1.5 to 2.5 per cent. (Note 1); together with varying quantities of magnesia, potash, and soda, making between them about 2.5 per cent. It is what is known to technologists as a hydraulic lime or mortar, i.e., one that possesses the valuable property of hardening under water, which it owes to the more or less intimate combination between the lime, silica, and alumina present.
The original of this now world-renowned building material was the so-called "Roman" cement, first made by mixing the volcanic tufa of Puzzuoli, near Naples, with ordinary slacked lime. This tufa in its composition closely resembles burnt clay, and hence the idea of mixing clay with chalk and then burning the mixture gradually developed in succession to the burning of natural calcareous stones, such as the septaria nodules of the London clay beds, from which the English-made Roman cement was first produced. This comparatively easy step was patented by Mr. Aspdin about the year 1824, who started works at Leeds, thus laying the foundations of the present enormous trade now principally centred round the valleys of the Thames and Medway (Note 2). The process as it at present exists may be briefly described as follows:—Chalk is mixed with the requisite quantity of clay in a "wash mill", a large circular basin of brickwork, in which a series of heavy iron drags or harrows are caused to rotate by the motion of a central spindle, to which they are suspended by radiating arms. Water is run in, and the two materials gradually disintegrate and thoroughly mix. The mixture, in the state of thin slop, known as " slurry", runs out of the mill as it is formed, and is led off to large settling tanks or ponds where it is allowed to remain at rest. The finely divided chalk and clay settle down to a stiff mass at the bottom, and the water is run off; a series of tanks being so arranged that each one is ready for subsequent treatment about six weeks after it has been filled. The stiff mass, or "compo", left behind when the water has run off, is in many works removed and ground in an ordinary mortar mill, after removal from which it is loaded on to drying floors which are heated, either with waste heat from kilns or coke ovens, or by special furnaces arranged for the purpose. A shed open at the sides protects the drying compo from rain, and it gradually solidifies into masses containing only a small percentage of moisture. These masses, varying from the size of a man's head to that of a fist, are placed in a kiln with alternate layers of coke. A fair sized kiln will hold 70 tons (Note 3) of dried slurry, 15 tons of coke, and a small quantity of brushwood to act as kindling.
The fire is now lighted, and by the fourth day, or in, say, ninety-six hours, the 85 tons of material have reached a temperature closely approaching that of molten cast iron, and have also diminished in weight to about 30 tons, plus the weight of the ashes of the coke (about 15 cwt.) (Note 4). The kiln requires a day to cool, another day to unload, and the 30 tons of hard cement clinker is ready to be crushed, and then ground into a marketable product. In addition to the above process, there are two modifications of the preliminary part of it in use, known respectively as the "semi dry" and "dry processes". These apply a less quantity of water in the mixing of the materials, the object being to do away with the settling tanks, and thus save space and labour. The treatment of the product in the kiln is, however, the same in all. It will be as well here to consider what has happened during the burning described above, and the first striking fact is the enormous decrease in weight. Firstly, the whole of the combustible portion of the coke has gone, mostly in the form of carbonic acid; secondly, the 70 tons of slurry has diminished to about 30 tons.
As far as the coke is concerned, assuming that 95 per cent. is carbon, the conversion of this into carbonic acid has involved the consumption of nearly 38 tons of oxygen, equal to nearly 166 tons of air; next the slurry has lost 40 tons, 38 tons being carbonic acid (Note 5), and the atmosphere has thus received from the kiln some 90 tons carbonic acid, 128 tons nitrogen, and about 2 tons of water in the shape of steam (Note 6). When the last of the coke has burned away, there is left in the kiln 30 tons of cement clinker at a temperature of over 2000 deg. Fah. (Note 7), the walls of the kiln have also, for some distance from the interior, been heated to the same degree, and before anything can be done this mass must have become cool enough for removal. It is obvious that the heat retained after the clinker is formed is lost during this cooling process. The value of this heat is considerable, and would probably be equal to nearly one-third of the total coke consumed (Note 8). In other words, it may be assumed that to reheat 30 tons clinker with the brickwork of the kiln would take five tons of coke. If this heat could be utilised it would effect a saving of, say, 2s. per ton of cement made. Another defect of the process arises from the large size of the lumps of slurry put in. Owing to this they invariably consist of (1) an over burnt exterior skin, (2) a properly burnt inner part, (3) if at all large, of an under burnt kernel (Note 9). All these are of necessity ground together; it is impossible to do more than make a very rough selection for the mill.
The clinker now cooled and ready for the mill is extremely hard (Note 10). It has first to be crushed by a Blake's or other crusher to lumps about the size of a walnut and these have to be ground under millstones. The extreme hardness of the material necessitates constant redressing of the stones to such an extent that at least a quarter of the stones in a cement mill are always " up " undergoing this process (Note 11). Thus, while the steam power required to deal with so refractory a material is a very heavy item, it is obvious that the maintenance of the mill in a thoroughly efficient state involves a constant and serious outlay. Another defect arises from the ash of the coke, which being inextricably mixed with the cement clinker, is therefore ground with it and acts so far as an adulterant (Note 12).
Returning to the kiln, we find that each firing involves the heating of the cold walls of the kiln to the necessary temperature, and again cooling them to the point at which it can be unloaded and again loaded up. The kilns have to be built of enormously massive masonry, to withstand the strains and racking caused by these constant violent changes of temperature. To keep them in repair involves very heavy outlay, and the first cost of a 30-ton kiln, of about 20ft. diameter and 35ft. to 40ft. high, with walls tapering from about 3ft., with its fire-brick lining, is extremely heavy (Note 13). The ground space occupied by it is also very large.
It will be seen from the above that the processes of burning and grinding the cement are by far the most costly of all the operations involved in its manufacture, and that they are beset with defects, both scientific and practical, of a very serious nature. It is evident that if any great improvement is to be effected in the manufacture, that to these portions of the process the most serious attention must be directed. It is therefore to this part of the work that Mr. Ransome has directed his attention; taking as his guiding principles economy of fuel, space, and labour, he has devised the following process.
The slurry prepared by any one of the methods now in use, is dried on a floor heated as usual or by waste gas from subsequent processes. The soft, friable, and easily-crushed blocks are now reduced to coarse powder, and are then ready for burning (Note 14). The old kiln is totally abolished, and in its place a cylinder of boiler plate is used. This is lined with good refractory fire-brick set in fire-clay, and about every fourth row bricks are set up on end, thus producing a number of parallel longitudinal feathers or ridges extending completely through the cylinder from end to end (Note 15). The outside of the cylinder is provided with two smooth rings or rails of iron. In the centre a third rail is wrought into teeth, into which a worm rotated at a slow speed gears. The two rails rest on friction rollers, and the whole cylinder being set at an angle with the horizon is caused to rotate slowly (Note 16). This construction, though sounding somewhat formidable, is in practice extremely simple, and similar machines, known as "black-ash revolvers", or "revolving black-ash furnaces", have long been, and are now, in daily use in alkali works (Note 17). The cylinder is mounted on the top of a brickwork chamber divided by interior walls of brick. The two outer chambers are filled with bricks piled in loosely, chequerwise, so as to present a large surface (Note 18).
We will now suppose a cylinder to be started, and describe the operations. A gas producer being in working order and delivering its gas at a regular rate, it is lighted and the flame passes through the cylinder, which in the course of a few hours attains a white heat. The waste heat from the revolver has also passed through and heated the right-hand division of the regenerator to a bright cherry red. A shunt valve is now opened, causing the waste gases to pass through the left-hand regenerator, while the gas from the producer is caused to flow through the heated right-hand chamber, and thus arrives at the mouth of the revolver already intensely heated. The result of this is that an immediate economy of fuel is produced, and to avoid overheat it will be necessary to reduce the gas supply. During the whole operation the air necessary for combustion is also heated by passing down a separate division of the regenerator, where it receives heat from the walls of the outer compartments. As soon as the right-hand chamber begins to cool, the furnaceman reverses his shunt valve and the fresh gas is turned through the hot regenerator, while the waste combustion products are heating that which has cooled down. The effect of this method of working is thus to return into the furnace the heat which in ordinary methods of work goes up the chimney. No startling innovation occurs save in the application of the method to cement making. Regenerative furnaces are in use all over the world, and an intelligent furnace-man will learn how to manage one in a few hours.
We have now to turn our attention to the cement which, taken from the drying floor, we described as crushed to a coarse powder. The powder is lifted by any convenient mechanical arrangement to a hopper, placed at the upper end of the revolver; from this it falls in a steady shower through the flame (Note 19), to the lower side of the cylinder, and lodges between the feathers. As the advancing side of the revolver rises it is lifted until the feather attains such an inclination as to shoot it off again through the flame to the bottom once more, but, owing to the incline, several inches nearer to the lower end. As the revolver moves on this operation continues again and again, the powder is constantly lifted and shot through the flame in showers, gradually getting nearer and nearer to the lower and hotter end of the cylinder, until at last it falls out into a receptacle at the lower end. In practice it is found desirable to rotate the cylinder at such a rate that any given particle of cement takes about thirty minutes to travel from one end to the other, during which time it has been lifted and shot through the flame about fifty times.
The powder has now arrived at the outside of the furnace, and having been delivered on to a floor to cool, is at once ready for grinding, that is, it is in the same state as the clinker after being seven days in the kiln. Unlike cement clinker, however (Note 20), it does not consist of lumps weighing from 14 lb. downward, and as hard as granite, but of a coarse, roughly agglutinated sand (Note 21). Nor does it consist of an overburnt skin, a properly burnt timer portion, and a possibly under-burnt inmost part, but if the operation has been properly carried out, each fragment has been heated to exactly the proper degree. How exactly this heat can be regulated is well known to all who have ever used a regenerative furnace. Again, the fuel used is gaseous, consequently no mixture of coke ash has taken place, and the cement is really and in fact what it professes to be.
So much would have been achieved had the new process been introduced with a furnace which had to be cooled in order to remove its contents, but - and here comes in a source of immense saving, not only in fuel, but in repairs — this is not an intermittent but a continuous one. The revolver once started goes on night and day delivering its hourly quantity of properly burnt cement until its firebrick lining requires renewing, an operation that only has to be performed occasionally. There is no constant loss of time and heat during cooling, loading, and unloading, as with the kiln, but the hourly delivery of a ton of cement enables a works with two cylinders to turn out 336 tons of cement per week, a quantity that eleven kilns of the usual capacity could not produce in the same time. As a matter of prudent practice the inventor advises that a spare cylinder with its regenerators should always be ready, so that a works using two cylinders would, as a fact, have three. In such a works, as soon as a revolver was seen to be in such a state as to require attention to its lining, gas would be turned on to the standing furnace, and in a few hours it could take up its duty, allowing the shut-down revolver to be repaired without any stoppage or diminution of the output. It is needless to say that the re-lining of a kiln is a very different matter, involving the loss of its services for many days.
The next question to be considered is the economy of fuel effected by the use of gas producers. Instead of consuming coke, these require only to be fed with slack, coal dust, or anything that will burn (Note 22), fed in at intervals through a hopper. A two-cylinder works would require for its daily service probably one gas producer, capable of converting about 6 cwt.. of slack per hour into gas. In addition, there should be one similar producer in reserve kept going at only one-fifth of its full power, its gas being utilised under the boiler or drying floors; it would thus always be ready in case of a breakdown to take up its full production and supply the revolvers in motion. These producers are chambers of brickwork, in which a portion of the fuel burning gasifies the rest, a small jet of steam being blown in to assist the operation. They consume the whole of the coal, nothing escaping but ashes, and thus alone effect a great saving in the stoppage of the waste of cinders inevitable under ordinary circumstances. Numerous first-rate makers exist, and the use of gas producers is daily extending in the country, even in places where coal is raised either on or in close proximity to the works. Their value as economisers is recognised in all furnace operations requiring intense local heats. In steel and glass-melting industries employing heats like that of the cement kiln, it is asserted that, coupled with the use of regenerators, a saving of 50 to 70 per cent of the fuel formerly used is effected. The only use for which their value is disputed is for steam raising, though in works where they are required for other purposes, even here they would effect savings in fire-bars and in wages. Their cost is small, they occupy little room, they can be placed at any reasonable distance from the place where the gas is to be burnt, so as to be in close proximity to the coal siding, any labourer can shovel the slack into them, and they do not require constant skilled supervision. As we have before stated, there are several forms in use, that of Wilson being one of the best.
On the occasion of my inspection of Mr. Ransome's experimental furnace at Grays, Essex, the revolver was furnished with gas from a small producer, built by the works' bricklayer, which was gasifying about 2 cwt. slack per hour; this not only supplied the furnace, but the valve was partly shut down to control the gas, which was, nevertheless, in excess of what was required. In fact. I felt convinced that the producer could have supplied two half-ton revolvers. There was accordingly here exhibited a consumption of about 2 cwt. slack per ton of cement, produced instead of the usual 7 to 10 cwt. coke per ton of cement clinker from the kiln. The results derived from this plan of gas firing are therefore:- (1) Possibility of working with regenerative furnaces thus saving all heat passing from the revolver. (2) Use of about 3 cwt. cheap slack per ton of cement instead of 7 cwt. coke. (3) Complete combustion of all fuel, the steam injected being decomposed by the red-hot cinders, and producing carbonic oxide and hydrogen. In all ordinary furnaces (Note 23) great quantities of fuel are lost, in the shape of cinders inextricably mixed with the ash or mineral matter of the coal. (4) The cement is kept entirely free from fuel ash.
In addition to these the revolver gives us the following advantages:— (1) Economy of space, two revolvers with their appurtenances, and one in reserve, covering 900 sq. ft., turning out the same weight of cement as eleven kilns covering 4400 sq. ft. (2) Continuous day and night working, and hence economy of fuel lost by necessary cooling, and subsequent re-heating of the kiln walls (Note 24). (3) Economy of repairs, which are simple and cheap (Note 25). (4) Less frequent need of repairs, as the continuous heat involves no racking like the alternate heating and cooling (Note 26). (5) Economy in first cost (Note 27). (6) Economy in grinding, a granular sand being produced instead of lumps of clinker, whereby crushers are quite abolished, and the wear and tear of the mill-stones greatly reduced (Note 28). (7) Economy of hand labour. Revolver cement can be handled on the American elevator system (Note 29). (8) Improved quality from (a) non-mixture with fuel ash; (b) no over-burning nor under burning. (9) Increased control over quality of cement, it being possible to stop, increase, or diminish the flow of crushed slurry and to vary its quality at any time. (10) Freedom from loss by accident. The ordinary kiln once charged and fired must burn out, whether charged wrongly or rightly, while, as before stated, any error in material can be rectified in a revolver as soon as discovered. (11) Perfect control of temperature (Note 30). And lastly (12) power of varying temperature according to nature of material. On the other side there are the inherent defects of the kiln process; which need not here be recapitulated, having been fully treated already, and, moreover, being indicated by contrast in the above summary. It will have been noticed in the description that the time occupied by the crushed slurry in passing through the cylinder is about half an hour. Compared with the time occupied by the kiln process, this will seem to the practical cement maker a very short — perhaps too short - a time to effect the necessary changes (Note 31). It must, however, be remembered that a very great part of the kiln operation is taken up in warming the large lumps of dried slurry, which, like all earths, are very bad conductors of heat. This is not needed in the revolver, as the coarse powder almost directly attains the heat of the cylinder. The small size of the particles also permits the rapid liberation of the carbonic acid from the chalk, and after that is done it is not necessary to keep the at heated, the necessary combination of the lime and clay taking place as soon as the particles are sufficiently hot, and this occurs long before the end of the cylinder is reached.
It must be remembered that a great part of the time taken in the kiln in necessary, by reason of its construction and the great mass of matter it contains, to the upper portions of which the fire can only reach after the lower parts have parted with most of their carbonic acid and moisture, which as they pass upward prevent the layers of coke above from burning. Much of the moisture also condenses in the upper part, only to be again driven out when the fire reaches it; this does not take place in the revolver, the gases rushing quickly past to the regenerator and meeting only a moderate quantity of cold powder on its way in. It is also almost needless to again point out that at least one-seventh of the time is taken up in loading, another seventh in cooling, and a third, together nearly half, in unloading. Of the remainder it is be observed that most of it is used in the gradual advance of the fire upwards, so that it may be safely stated that the lower layers of the kiln are sufficiently burnt a few hours after the start, and from then to the complete burning of the upper part they are simply lying idle. Could they be removed, as is the case in the revolver, they might be at once ground; but this removal is, from the nature of the method, impossible.
In addition to the method of burning just described, Mr. Ransome has introduced another improvement, which, however, is available only in certain districts. This is the introduction of a new material in cement making in the form of blast-furnace slag. This is, as is well known, produced in enormous quantities, and notwithstanding innumerable attempts to utilise it, it is in truth a valueless waste material, costing the ironmaster large sums either for carriage to sea or for land to deposit it on. The ores used mostly for the production of iron consist of the carbonate of that metal mixed with silica and alumina. The latter are removed by the addition of lime, in the shape of limestone, to the furnace charge. The lime combines with the silica and alumina to form a fusible substance. This continually runs from the furnace and constitutes the slag in question. It contains the same elements as Portland cement, though in different proportions, as the following analyses will show (Note 32):
| Middlesbrough slags | |||
|---|---|---|---|
| Ferrous oxide | 0.72 | 3.64 | 0.61 |
| Manganous oxide | 0.35 | 1.02 | trace |
| Alumina | 24.69 | 20.72 | 22.28 |
| Lime | 40.00 | 36.88 | 40.45 |
| Magnesia | 3.55 | 4.25 | 7.21 |
| Silica | 27.65 | 30.40 | 27.80 |
| Potash | 0.46 | 0.50 | 0.00 |
| Soda | 0.99 | 0.00 | 0.00 |
| Sulphur | 1.95 | 1.34 | 2.00 |
| Phosphorus | 0.26 | 0.00 | 0.00 |
| Portland cements | ||
|---|---|---|
| Ferric oxide | 3.41 | 5.46 |
| Alumina | 6.92 | 8.00 |
| Lime | 59.90 | 55.57 |
| Magnesia | 0.82 | 0.77 |
| Silica | 24.07 | 22.92 |
| Potash | 0.73 | 1.13 |
| Soda | 0.87 | 1.70 |
| Sulphur | 0.67 | 0.41 |
Neglecting the non-essential constituents, we have then a body containing silica in nearly the same proportion as does cement, lime in nearly two-thirds the proportion, and alumina in nearly a threefold proportion. If it be admitted that the functions of the alumina and silica in cement are reciprocal (Note 33), or nearly so, it follows that we may add these substances together, when we shall have the following results, again, for the sake of clearness, omitting the non-essential substances:—
| Middlesbrough slags | Portland cements | ||||
|---|---|---|---|---|---|
| Alumina and silica | 52.34 | 51.12 | 50.08 | 30.99 | 30.92 |
| Lime | 40.00 | 36.88 | 40.45 | 59.90 | 55.57 |
From this it will be seen that in the slag the ratio of lime to the two other bodies is about 39 : 51, while in the cement it is 58 : 31. A simple calculation will show that on this basis the 39 + 51 = 90, or, including the other bodies, 100 parts of slag requires the addition of 56 parts of lime = 100 parts of calcium carbonate, i.e., dry chalk or limestone, to give a substance yielding a good cement (Note 34). The only difficulty is the hardness of the slag, but this is overcome by Mr. C. Wood's method of running it into water, when it disintegrates, and yields a slag sand, which is easily ground with the chalk after separation of a little entangled iron by sifting. Attempts have long since been made to produce this cement in the ordinary kiln, but have been abandoned, as the mixture has no coherence, and the lumps fell to pieces as soon as they got hot, and choked up the draught, putting the fire out (Note 35).
This friability, disastrous in the kiln, is no defect but rather virtue in the revolver, so that in this matter the two inventions supplement each other, and the revolver thus brings a new cement material to the fore. It must, of course, be understood that slag cement cannot be made to compete with chalk-clay cement except in the neighbourhood of iron works. In the South of England the cost of carriage of the slag prevents it from being used, as clay is to be had on the spot. There is, however, every reason to believe that the slag cement may prove a profitable manufacture in the iron districts where the cement revolvers could themselves be fired by means of the blast furnace gases which are now everywhere being utilised as fuel.
Careful tests of the new material have been made, from which it would appear that slag cement attains its strength more rapidly than does ordinary Portland, for it was found that Ransome's had a breaking strain of 1440 lb. on an area of 2¼ square inches in twenty-eight days, while the Portland reached 1325 lb. only in two years. The result is even more striking if short periods are taken, as the following shows (Note 36):—
| Days | Portland, lb per 2¼ square inch area, 123 lb. per bushel. | Ransome's slag cement, lb per 2¼ square inch area, 129 lb. per bushel. |
|---|---|---|
| 2 | 510 | 740 |
| 3 | 698 | 870 |
| 7 | 818 | 1170 |
It is therefore evident that this process is well worth the attention of cement makers in our iron districts, while the revolver process of burning must before long come into universal use.
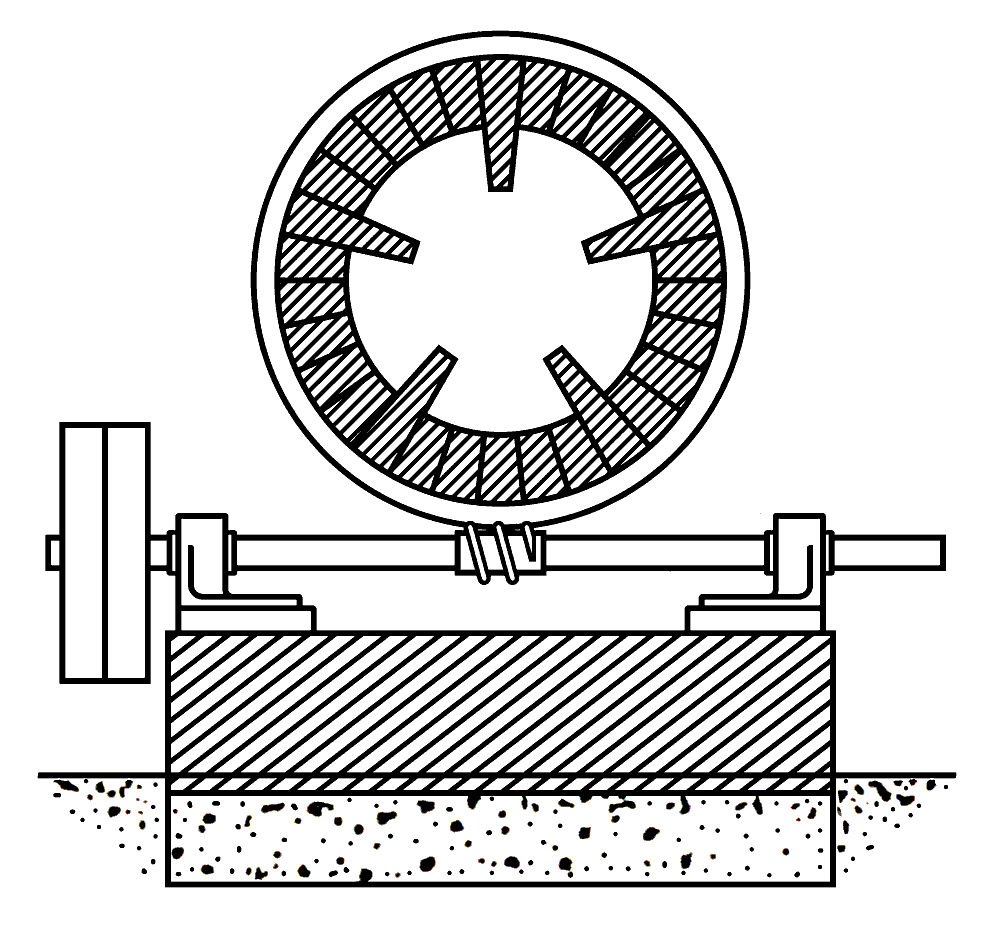 Cross section of kiln from the 3rd edition of Ransome's patent.
Cross section of kiln from the 3rd edition of Ransome's patent.